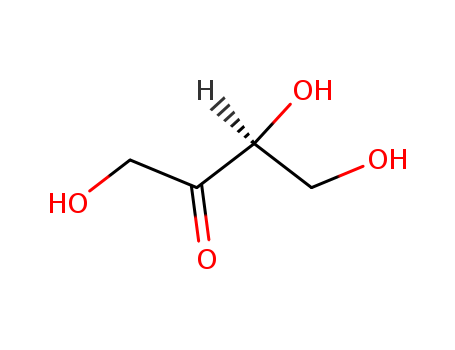
533-50-6
- Product Name:L-Erythrulose
- Molecular Formula:C4H8O4
- Purity:99%
- Molecular Weight:120.105
Product Details
Perfect Factory Offer Excellent quality L-Erythrulose 533-50-6 with Safe Shipping
- Molecular Formula:C4H8O4
- Molecular Weight:120.105
- Vapor Pressure:2.8E-06mmHg at 25°C
- Refractive Index:1.507
- Boiling Point:349.6 °C at 760 mmHg
- PKA:12.00±0.20(Predicted)
- Flash Point:179.4 °C
- PSA:77.76000
- Density:1.42g/cm3
- LogP:-2.09900
L-(+)-Erythrulose(Cas 533-50-6) Usage
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products, air care products, biocides (e.g. disinfectants, pest control products), perfumes and fragrances, polishes and waxes and washing & cleaning products. Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid. |
InChI:InChI=1/C4H8O4/c5-1-3(7)4(8)2-6/h3,5-7H,1-2H2/t3-/m0/s1
533-50-6 Relevant articles
Microfluidic multi-input reactor for biocatalytic synthesis using transketolase
Lawrence, James,O'Sullivan, Brian,Lye, Gary J.,Wohlgemuth, Roland,Szita, Nicolas
, p. 111 - 117 (2013)
Biocatalytic synthesis in continuous-flo...
L-erythrulose production by oxidative fermentation is catalyzed by PQQ-containing membrane-bound dehydrogenase.
Moonmangmee, Duangtip,Adachi, Osao,Shinagawa, Emiko,Toyama, Hirohide,Theeragool, Gunjana,Lotong, Napha,Matsushita, Kazunobu
, p. 307 - 318 (2002)
Thermotolerant Gluconobacter frateurii C...
PROCESSES FOR PREPARING C-4 SUGARS AND KETOSE SUGARS
-
Page/Page column 37-39, (2021/11/20)
Various processes for preparing C4 aldos...
Enantioselective Reductive Oligomerization of Carbon Dioxide into l-Erythrulose via a Chemoenzymatic Catalysis
Bontemps, Sébastien,Clapés, Pere,Desmons, Sarah,Dumon, Claire,Fauré, Régis,Grayson-Steel, Katie,Hurtado, John,Nu?ez-Dallos, Nelson,Vendier, Laure
supporting information, p. 16274 - 16283 (2021/10/12)
A cell-free enantioselective transformat...
D -Serine as a Key Building Block: Enzymatic Process Development and Smart Applications within the Cascade Enzymatic Concept
Auffray, Pascal,Charmantray, Franck,Collin, Jér?me,Hecquet, Laurence,L'Enfant, Mélanie,Martin, Juliette,Ocal, Nazim,Pollegioni, Loredano
, p. 769 - 775 (2020/07/14)
An efficient enzymatic method catalyzed ...
One-Pot Cascade Synthesis of (3S)-Hydroxyketones Catalyzed by Transketolase via Hydroxypyruvate Generated in Situ from d-Serine by d-Amino Acid Oxidase
L'enfant, Mélanie,Bruna, Felipe,Lorillière, Marion,Ocal, Nazim,Fessner, Wolf-Dieter,Pollegioni, Loredano,Charmantray, Franck,Hecquet, Laurence
, p. 2550 - 2558 (2019/04/17)
We described an efficient in situ genera...
533-50-6 Process route
-
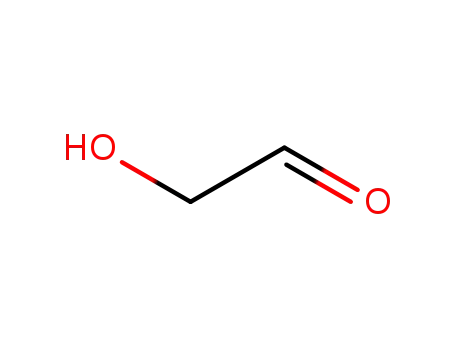
- 141-46-8
Glycolaldehyde

-
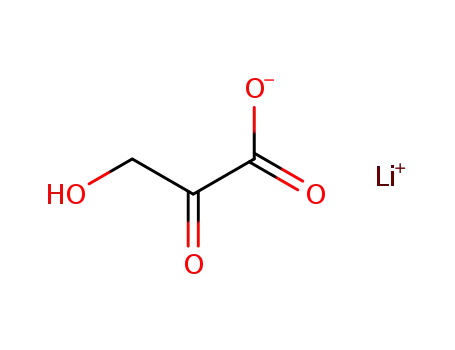
- 3369-79-7
Lithium hydroxypyruvate

-
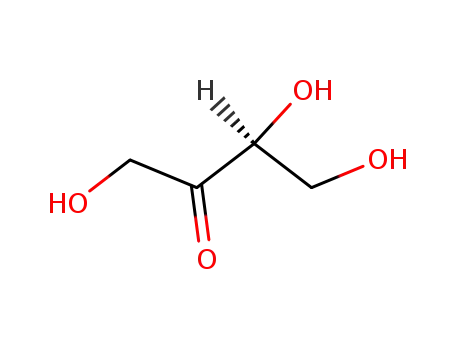
- 533-50-6
L-erythrulose
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; magnesium(II) chloride hexahydrate; thiamine diphosphate; In water; at 25 ℃; for 24h; pH=7; optical yield given as %ee; stereoselective reaction; Enzymatic reaction;
|
60% |
|
With thiamine diphosphate; sodium hydroxide; magnesium chloride; In glycylglycine buffer; at 25 ℃; for 0.5h; pH=7.5; Enzymatic reaction;
|
56% |
|
With magnesium(II) chloride hexahydrate; trans ketolase from Geobacillus stearothermophilus; thiamine diphosphate; sodium hydroxide; In water; at 50 ℃; for 0.333333h; pH=7.5; stereospecific reaction; Enzymatic reaction;
|
44% |
|
With magnesium chloride hexahydrate; Escherichia coli wild-tipe transketolase; thiamine diphosphate; sodium hydroxide; In water; at 25 ℃; for 24h; pH=7; stereoselective reaction;
|
|
|
With D469E transketolase; thiamine diphosphate; magnesium chloride; at 20 ℃; pH=7; aq. buffer; Enzymatic reaction;
|
|
|
With thiamine diphosphate; magnesium chloride; for 2h; pH=7; optical yield given as %ee; aq. buffer; Enzymatic reaction;
|
|
|
With thiamine diphosphate; magnesium chloride; pH=7; Enzymatic reaction;
|
|
|
With Saccharomyces cerevisiae transketolase; thiamine pyrophosphate; magnesium(II); In aq. phosphate buffer; at 25 ℃; pH=7; Enzymatic reaction;
|
-
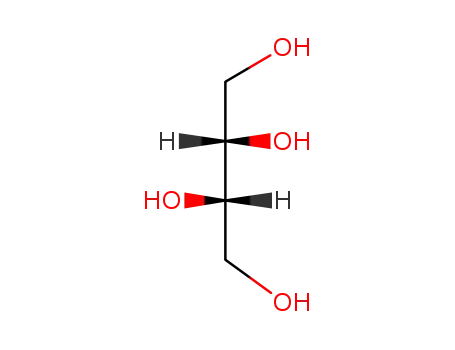
- 2319-57-5,10030-58-7,188346-77-2,149-32-6
threitol

-

- 533-50-6
L-erythrulose
| Conditions | Yield |
|---|---|
|
With [(neocuproine)Pd(OAc)]2(OTf)2; oxygen; In water; acetonitrile; at 25 ℃; for 20h; chemoselective reaction;
|
86% |
533-50-6 Upstream products
-
186581-53-3

diazomethane
-
73945-73-0
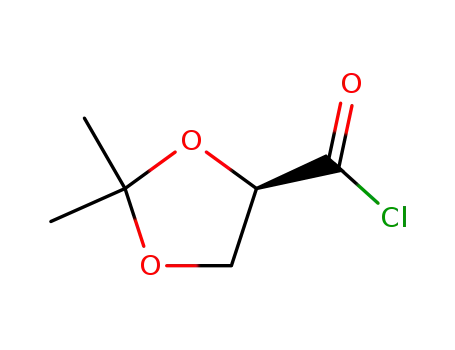
(R)-2,3-o-isopropylidene-D-glyceroyl chloride
-
583-50-6
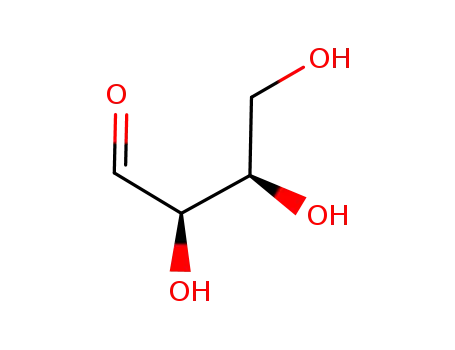
D-erythrose
-
95-43-2
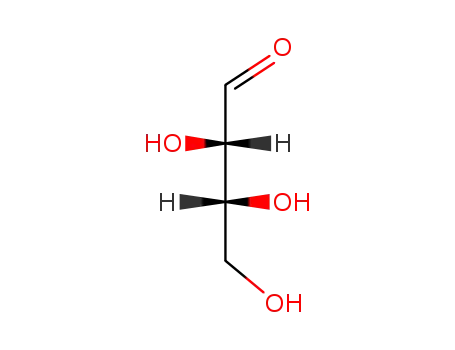
D-threose
533-50-6 Downstream products
-
95-43-2

D-threose
-
583-50-6

D-erythrose
-
57-55-6
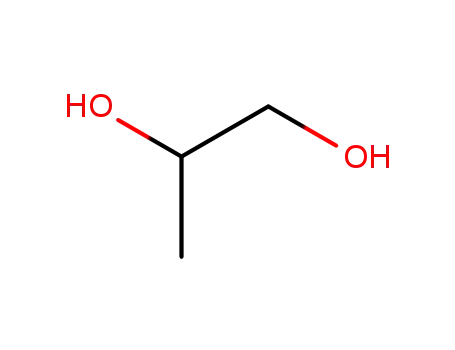
propylene glycol
-
2418-52-2
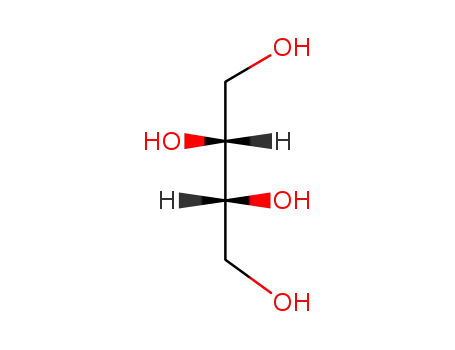
D-threitol
Relevant Products
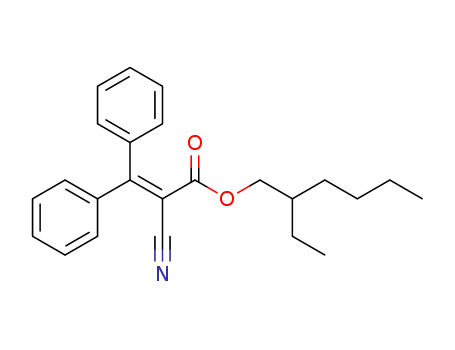
-
0ctocrylene
CAS:6197-30-4
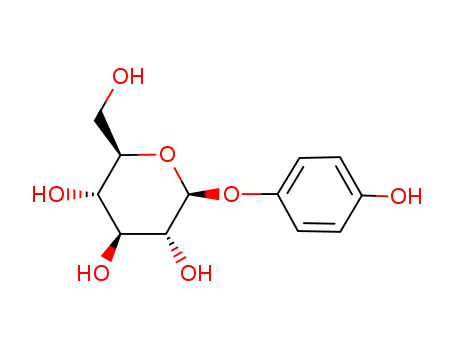
-
Beta Arbutin
CAS:497-76-7
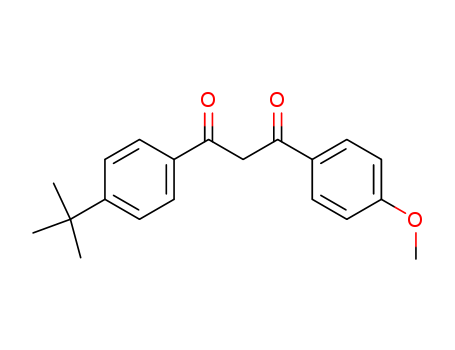
-
Avobenzone
CAS:70356-09-1