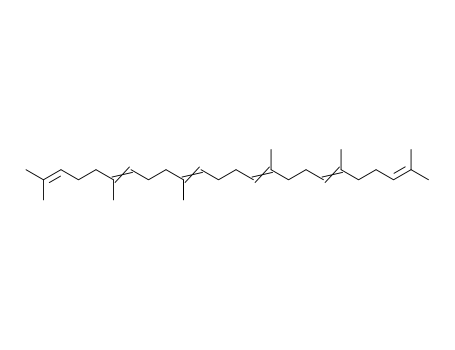
Product Details
pd_meltingpoint:-75 ºC
Appearance:Clear, slightly yellow liquid with a faint odor.
Quality Factory Sells Top Purity 99% Squalene 111-02-4 with Safe Delivery
- Molecular Formula:C30H50
- Molecular Weight:410.727
- Appearance/Colour:Clear, slightly yellow liquid with a faint odor.
- Vapor Pressure:0mmHg at 25°C
- Melting Point:-75 ºC
- Refractive Index:n20/D 1.494(lit.)
- Boiling Point:285 ºC (25 mmHg)
- Flash Point:254.1°C
- PSA:0.00000
- Density:0.858
- LogP:10.60500
Squalene(Cas 111-02-4) Usage
|
Air & Water Reactions |
May become discolored on exposure to air. Insoluble in water. |
|
Reactivity Profile |
(all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is incompatible with strong oxidizing agents. . |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene emits toxic fumes of carbon monoxide and carbon dioxide. |
|
Fire Hazard |
(all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
Squalene is a biosynthetic precursor to all steroids. It acts as a cytoprotective agent to normal cells exposed to carcinogens and antitumor agents. Squalene helps in equalizing the blood cholesterol levels. It increases the production of HDL (high density lipoprotein) and the excretion of LDL (low density lipoprotein). This helps in reducing the risk of heart disease and protects the less stable body fats from oxidation. It is also used in treating hypercholesterolemia. Squalene is known to improve the efficiency of cholesterol lowering drugs. It also serves as an antioxidant by preventing the effects of free radical. It protects skin from drying and other environmental conditions such as oxidation, ultraviolet rays and pollutants. Squalene is known to promote wound healing. |
|
Definition |
ChEBI: A triterpene consisting of 2,6,10,15,19,23-hexamethyltetracosane having six double bonds at the 2-, 6-, 10-, 14-, 18- and 22-positions with (all-E)-configuration. |
|
General Description |
Clear, slightly yellow liquid with a faint odor. Density 0.858 g / cm3. |
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products. Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid. |
InChI:InChI=1/C30H50/c1-25(2)15-11-19-29(7)23-13-21-27(5)17-9-10-18-28(6)22-14-24-30(8)20-12-16-26(3)4/h15-18,23-24H,9-14,19-22H2,1-8H3/b27-17+,28-18+,29-23+,30-24+
111-02-4 Relevant articles
Application of the chloro ketal Claisen reaction to the total synthesis of squalene.
Werthemann,Johnson
, (1970)
-
Mutated variants of squalene-hopene cyclase: Enzymatic syntheses of triterpenes bearing oxygen-bridged monocycles and a new 6,6,6,6,6-fusded pentacyclic scaffold, named neogammacerane, from 2,3-oxidosqualene
Fukuda, Yoriyuki,Watanabe, Takashi,Hoshino, Tsutomu
, p. 6987 - 7000 (2018)
Squalene-hopene cyclase (SHC) catalyzes ...
Cloning, expression analysis and functional characterization of squalene synthase (SQS) from tripterygium wilfordii
Zhang, Bin,Liu, Yan,Chen, Mengmeng,Feng, Juntao,Ma, Zhiqing,Zhang, Xing,Zhu, Chuanshu
, (2018)
Celastrol is an active triterpenoid comp...
Enzymic products of the 2,3-oxidosqualene analog having an ethyl residue at 10-position. First trapping of the trimethylcyclohexanone ring by lanosterol synthase
Hoshino, Tsutomu,Sakai, Yoshiyuki
, p. 7319 - 7323 (2001)
Incubation of squalene analog, (3RS)-(6E...
-
Isler et al.
, (1958)
-
Squalene Synthetase. Inhibition by Ammonium Analogues of Carbocationic Intermediates in the Conversion of Presqualene Diphosphate to Squalene
Poulter, C. Dale,Capson, Todd L.,Thompson, Michael D.,Bard, Ronda S.
, p. 3734 - 3739 (1989)
Squalene synthetase (EC 2.5.1.21) cataly...
-
Bhalerao,U.T.,Rapoport,H.
, p. 5311 - 5313 (1971)
-
ent-Kaurene and squalene synthesis in Fusarium fujikuroi cell-free extracts
Fernandez-Martin, Rafael,Domenech, Carlos,Cerda-Olmedo, Enrique,Avalos, Javier
, p. 723 - 728 (2000)
Sterols and gibberellins are the main te...
Reductive coupling of terpenic allylic halides catalyzed by Cp 2TiCl: A short and efficient asymmetric synthesis of onocerane triterpenes
Barrero, Alejandro F.,Herrador, M. Mar,Del Moral, Jose F. Quilez,Arteaga, Pilar,Arteaga, Jesus F.,Piedra, Maria,Sanchez, Elena M.
, p. 2301 - 2304 (2005)
(Chemical Equation Presented) Titanocene...
PALLADIUM-CATALYZED COUPLING OF ALLYLIC ACETATES WITH ZINC
Sasaoka, Shin-ichi,Yamamoto, Taku,Kinoshita, Hideki,Inomata, Katsuhiko,Kotake, Hiroshi
, p. 315 - 318 (1985)
Allylic acetates were coupled with zinc ...
-
Axelrod,E.H. et al.
, p. 2139 - 2141 (1970)
-
-
Karrer
, p. 130 (1953)
-
Hexacarbonylmolybdenum(0)-Catalyzed Reductive Coupling of Allylic Acetates
Masuyama, Yoshiro,Otake, Kiyotaka,Kurusu, Yasuhiko
, p. 1527 - 1528 (1987)
The reaction of allylic acetates with zi...
SYNTHESIS OF E,E-FARNESOL, FARNESYL ACETATE AND SQUALENE FROM FARNESENE VIA FARNESYL CHLORIDE
-
Paragraph 0090, (2019/12/28)
The present disclosure provides methods ...
Homocoupling versus reduction of radicals: An experimental and theoretical study of Ti(iii)-mediated deoxygenation of activated alcohols
Prieto, Consuelo,González Delgado, José A.,Arteaga, Jesús F.,Jaraíz, Martín,López-Pérez, José L.,Barrero, Alejandro F.
, p. 3462 - 3469 (2015/03/18)
A detailed experimental and theoretical ...
111-02-4 Process route
-
-
C36H54O2S

-
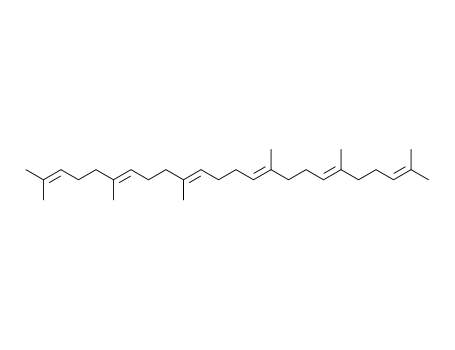
- 111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene
| Conditions | Yield |
|---|---|
|
With bis(diphenylphosphino)propanepalladium(II) dichloride; lithium triethylborohydride; In tetrahydrofuran; at -78 - 20 ℃; for 50h;
|
88.7% |
-
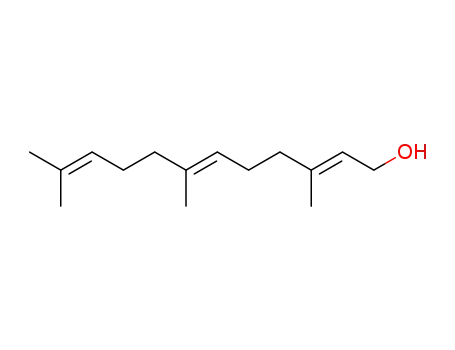
- 106-28-5
Farnesol

-

- 111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene
| Conditions | Yield |
|---|---|
|
Farnesol; With tert.-butyl lithium; In tetrahydrofuran; n-heptane; at -78 - 20 ℃; for 0.333333h; Inert atmosphere;
With bis(cyclopentadienyl)titanium dichloride; manganese; In tetrahydrofuran; for 10h; Inert atmosphere; Reflux;
|
67% |
|
Multi-step reaction with 2 steps
1: PBr3 / tetrahydrofuran / 0.5 h / 0 °C
2: CuI; pyrrolidine; n-BuLi / diethyl ether / 1 h / -38 °C
With pyrrolidine; copper(l) iodide; n-butyllithium; phosphorus tribromide; In tetrahydrofuran; diethyl ether;
|
|
|
Multi-step reaction with 2 steps
1: PBr3 / tetrahydrofuran / 0.5 h / 0 °C
2: CuI; pyrrolidine; n-BuLi / diethyl ether / 1 h / -38 °C
With pyrrolidine; copper(l) iodide; n-butyllithium; phosphorus tribromide; In tetrahydrofuran; diethyl ether;
|
|
|
Multi-step reaction with 3 steps
1: triethylamine / CH2Cl2 / 0.5 h / -40 °C
2: lithium bromide / CH2Cl2; tetrahydrofuran / 1 h / 0 °C
3: 1) lithium biphenylide, barium iodide / 1) THF, -78 deg C, 30 min, 2) THF, a) -78 deg C, 3 h, b) 23 deg C, 16 h
With lithium biphenylide; barium(II) iodide; triethylamine; lithium bromide; In tetrahydrofuran; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: triethylamine / CH2Cl2 / 0.5 h / -40 °C
2: lithium chloride / CH2Cl2; tetrahydrofuran / 1 h / 0 °C
3: 1) lithium biphenylide, barium iodide / 1) THF, -78 deg C, 30 min, 2) THF, a) -78 deg C, 3 h, b) 23 deg C, 16 h
With lithium biphenylide; barium(II) iodide; triethylamine; lithium chloride; In tetrahydrofuran; dichloromethane;
|
111-02-4 Upstream products
-
110-52-1
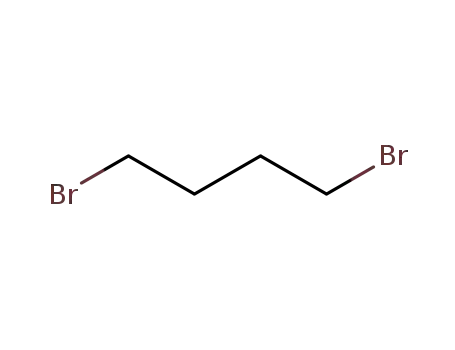
1,4-dibromo-butane
-
689-67-8
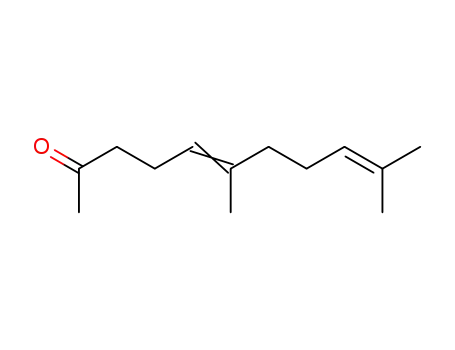
pseudo-ionone
-
24163-93-7

farnesyl bromide
-
60-29-7
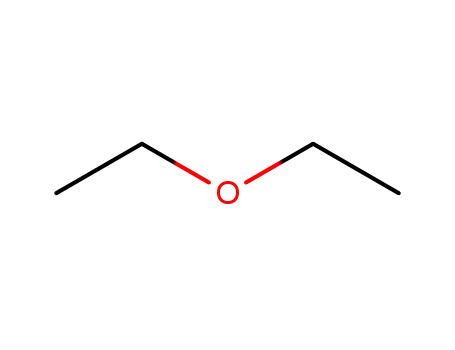
diethyl ether
111-02-4 Downstream products
-
513-35-9
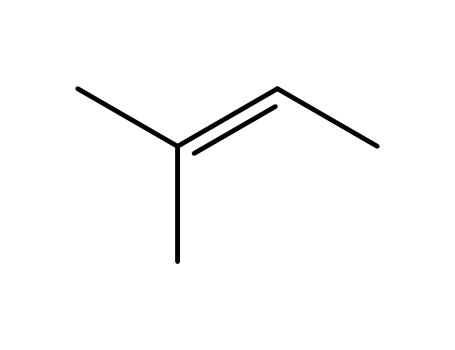
2-methyl-but-2-ene
-
1604-34-8
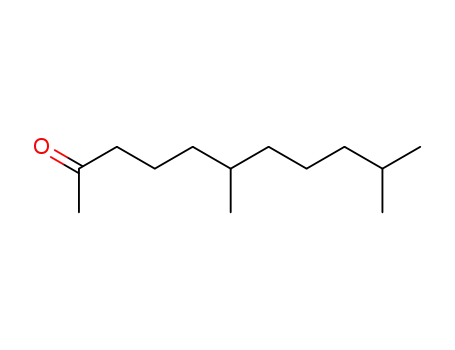
6,10-dimethyl-undecan-2-one
-
110-93-0
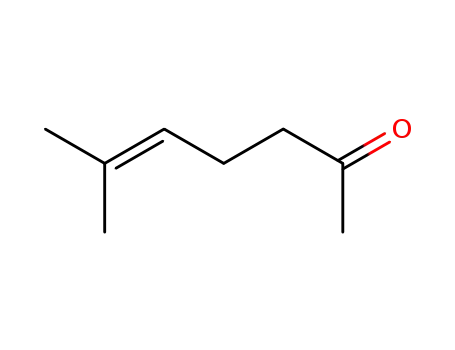
6-Methyl-hept-5-en-2-on
-
646-07-1
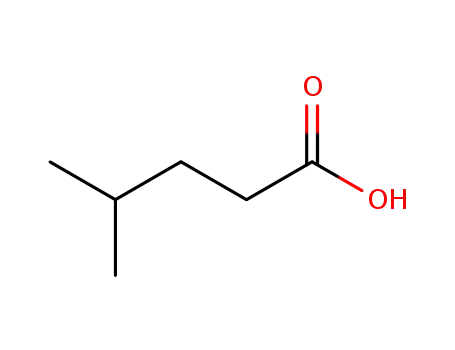
4-Methylpentanoic acid
Relevant Products
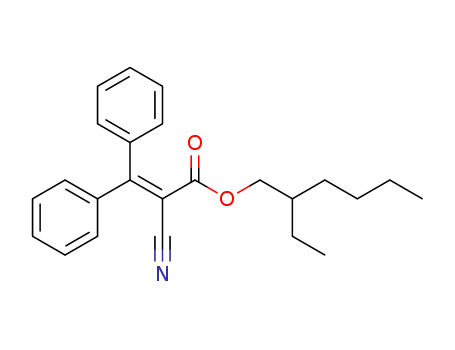
-
0ctocrylene
CAS:6197-30-4
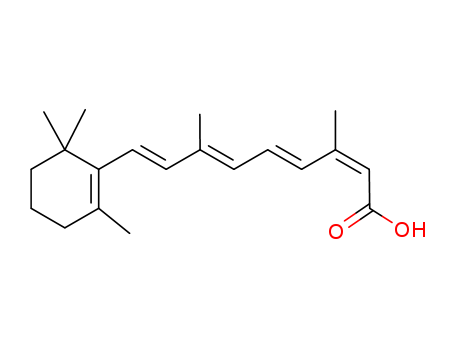
-
Isotretinoin
CAS:4759-48-2
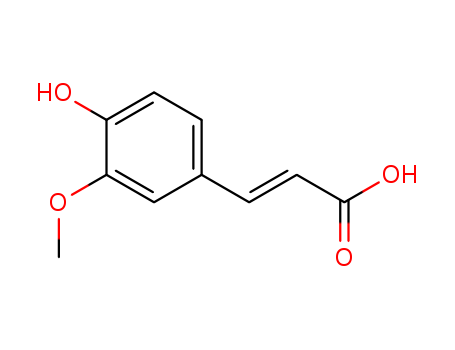
-
Ferulic Acid
CAS:1135-24-6