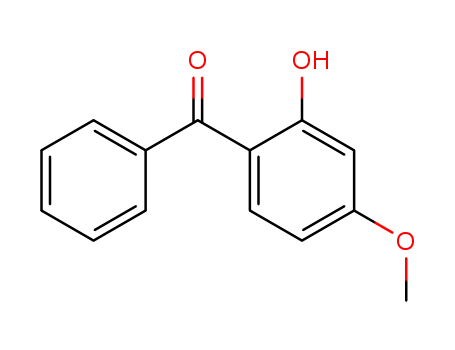
131-57-7
- Product Name:Benzophenone-3
- Molecular Formula:C14H12O3
- Purity:99%
- Molecular Weight:228.247
Product Details
pd_meltingpoint:62-64 °C(lit.)
Appearance:white to light yellow crystalline powder
Buy Good Quality Benzophenone-3 131-57-7 with a minimum purity of 99%
- Molecular Formula:C14H12O3
- Molecular Weight:228.247
- Appearance/Colour:white to light yellow crystalline powder
- Vapor Pressure:5.26E-06mmHg at 25°C
- Melting Point:62-64 °C(lit.)
- Refractive Index:1.595
- Boiling Point:370.3 °C at 760 mmHg
- PKA:7.56±0.35(Predicted)
- Flash Point:140.5 °C
- PSA:46.53000
- Density:1.201 g/cm3
- LogP:2.63180
Oxybenzone(Cas 131-57-7) Usage
|
Application and Synthesis |
UV-9 is a light yellow or white crystalline powder, also known as sunscreen 2, BP-3, suitable for polyvinyl chloride, polyvinylidene chloride, polymethyl methacrylate , unsaturated polyester, ABS resin and cellulose resin and other plastics, the maximum absorption wavelength range of 280-340 nm, the general amount of 0.1-1.5%, good thermal stability, does not decompose at 200 ℃. This product hardly absorb visible light, it applies to light-colored transparent products. This product can also be used for paints and synthetic rubber. For the broad-spectrum UV absorbers, with high absorption rate, non-toxic, non-teratogenic effect, light, thermal stability. UV-9 can absorb UV-A and UV-B together, is the US FDA approved Class I sunscreen and being used with a higher frequency in the US and European countries. UV-9 is widely used in sunscreen cream, cream, honey, lotion, oil and other sunscreen cosmetics, but also as a product from the photosensitive and discoloration of the anti-discoloration agent. In addition, it is usually used for PVC and unsaturated polyester and many other fields, because UV-9 has good compatibility with many kinds of plastics and resins. |
|
Improvement of UV - 9 Production Process |
Using mixed acid as catalyst, trichloromethylbenzene and resorcinol were condensed into intermediate 2,4-dihydroxydiphenyl ketone in aqueous phase, and then the catalyst was treated with phase transfer catalyst, etherification of the target product UV-9. |
|
Preparation |
Preparation by partial methylation of 2,4- dihydroxy-benzophenone, ? with methyl iodide in the presence of sodium hydroxide; ? with a methyl halide; ? with dimethyl sulfate in the presence of sodium hydroxide or alkaline solution. |
|
Synthesis Reference(s) |
Tetrahedron, 42, p. 885, 1986 DOI: 10.1016/S0040-4020(01)87495-0 |
|
Air & Water Reactions |
Insoluble in water. |
|
Fire Hazard |
Flash point data for Oxybenzone are not available; however, Oxybenzone is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Contact allergens |
BZP-3 is used as a direct sunscreen agent and in antiaging creams. Allergic reactions have been reported. Cross-reactivity is expected in an average of one in four patients photoallergic to ketoprofen. |
|
Safety Profile |
Poison by intraperitoneal route. Mddly toxic by ingestion. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Chemical Properties and Functionality |
Oxybenzone (benzophenone-3) is an organic UV protectant added to many sunscreen products. It absorbs UV photons and dissipates their energy as harmless heat, protecting against the damaging effects of ultraviolet light. Found in approximately 3,500 sunscreen products worldwide. |
|
Safety and Regulatory Approval |
Approved for use in sunscreen products by regulatory authorities in the United States, Canada, and the European Union. |
|
Skin Sensitivity Concerns |
Oxybenzone can induce photosensitivity dermatitis, characterized by skin redness, rash, and itching after sun exposure. Severe cases may result in facial swelling, requiring medical treatment. |
|
Environmental Impact |
Oxybenzone can contribute to coral reef damage, affecting coral growth and causing coral bleaching. Some areas have phased out oxybenzone-containing sunscreens due to concerns about coral reef health. |
|
description |
Oxybenzone is an organic compound used in sunscreens. It is a derivative of benzophenone. It forms colorless crystals that are readily soluble in most organic solvents. It is used as an ingredient in sunscreen and other cosmetics because it absorbs UV-A ultraviolet rays.Oxybenzone absorbs UVB and UVA II rays, resulting in a photochemical excitation and absorption of energy. Upon return to ground state, the absorbed energy results in emission of longer wavelength radiation and decreased skin penetration of radiation which reduces the risk of DNA damage. |
|
Chemical properties |
light yellow crystalline powder, soluble in ethanol, acetone and other organic solvents, insoluble in water. |
|
Definition |
ChEBI: A hydroxybenzophenone that is benzophenone which is substituted at the 2- and 4-positions of one of the benzene rings by hydroxy and methoxy groups respectively. |
|
Brand name |
Uvinul M40 (BASF). |
|
General Description |
White to off-white or light yellow powder. |
InChI:InChI=1/C14H12O3/c1-17-11-7-8-12(13(15)9-11)14(16)10-5-3-2-4-6-10/h2-9,15H,1H3
131-57-7 Relevant articles
Synthesis, analgesic, anti-inflammatory, COX/5-LOX inhibition, ulcerogenic evaluation, and docking study of benzimidazole bearing indole and benzophenone analogs
Khamees, Hussien Ahmed,Khanum, Shaukath Ara,Nagesh, Khadri M. J.,Prashanth, T.
, (2022/03/16)
Inflammation therapy is particularly foc...
Preparation method of sun-screening agent 2-hydroxy-4-methoxy-5-sulfo benzophenone
-
Paragraph 0052-0053, (2021/02/06)
The invention provides a preparation met...
Aerobic Copper-Catalyzed Salicylaldehydic Cformyl?H Arylations with Arylboronic Acids
Xiao, Lin,Lang, Tao-Tao,Jiang, Ying,Zang, Zhong-Lin,Zhou, Cheng-He,Cai, Gui-Xin
supporting information, p. 3278 - 3283 (2021/02/01)
We report a challenging copper-catalyzed...
Selective Oxidation of Alkylarenes to the Aromatic Ketones or Benzaldehydes with Water
Du, Jihong,Duan, Baogen,Liu, Kun,Liu, Renhua,Yu, Feifei,Yuan, Yongkun,Zhang, Chenyang,Zhang, Jin
supporting information, (2022/02/09)
Here a palladium-catalyzed oxidation met...
131-57-7 Process route
-
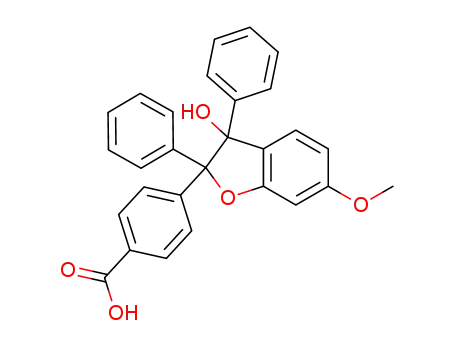
-
4-(3-hydroxy-6-methoxy-2,3-diphenyl-2,3-dihydrobenzofuran-2-yl)-benzoic acid

-
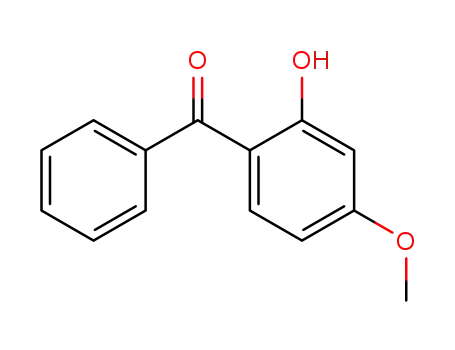
- 131-57-7,897050-18-9
Benzophenone-3

-
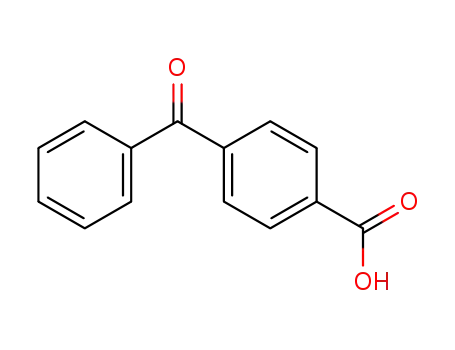
- 611-95-0
4-carboxybenzophenone
| Conditions | Yield |
|---|---|
|
With air; In water; acetonitrile; for 1h; Solvent; Irradiation;
|
21.7% 21.1% |
-
![methyl 4-[(2-benzoyl-5-methoxyphenoxy)(phenyl)methyl]benzoate](/upload/2024/7/eed372b7-a7b8-4ce6-b60d-2fdfa7693fbe.png)
-
methyl 4-[(2-benzoyl-5-methoxyphenoxy)(phenyl)methyl]benzoate

-

- 131-57-7,897050-18-9
Benzophenone-3

-

- 611-95-0
4-carboxybenzophenone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: sodium hydroxide; water / tetrahydrofuran / 22 h / 20 °C
2: Irradiation
3: air / acetonitrile; water / 1 h / Irradiation
With water; sodium hydroxide; In tetrahydrofuran; water; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: dichloromethane; cyclohexane / 1.5 h / Photolysis
2: sodium hydroxide; water / tetrahydrofuran / 22 h / 20 °C
3: air / acetonitrile; water / 1 h / Irradiation
With water; sodium hydroxide; In tetrahydrofuran; dichloromethane; cyclohexane; water; acetonitrile;
|
131-57-7 Upstream products
-
3555-84-8
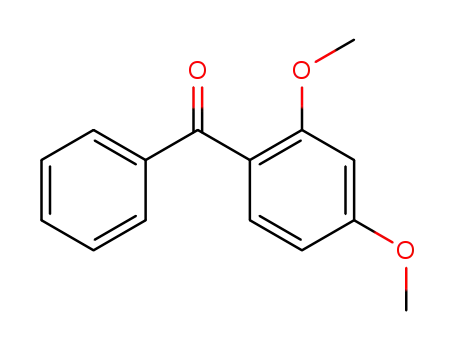
2,4-dimethoxybenzophenone
-
98-88-4
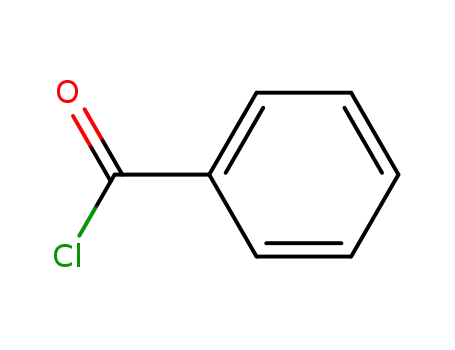
benzoyl chloride
-
151-10-0
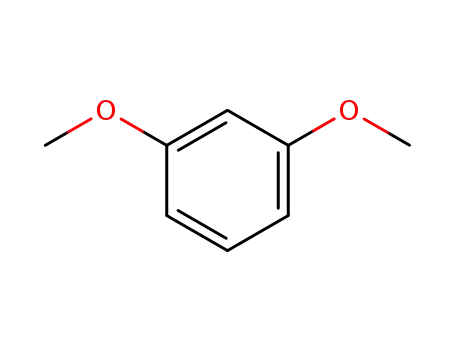
1,3-Dimethoxybenzene
-
131-56-6
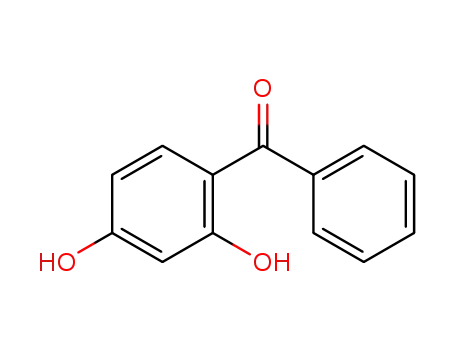
2.4-dihydroxybenzophenone
131-57-7 Downstream products
-
52811-37-7
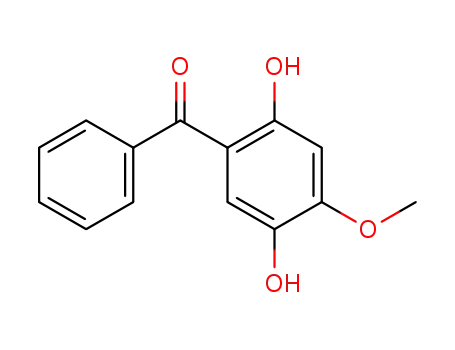
(2,5-dihydroxy-4-methoxyphenyl)phenylmethanone
-
2555-31-9
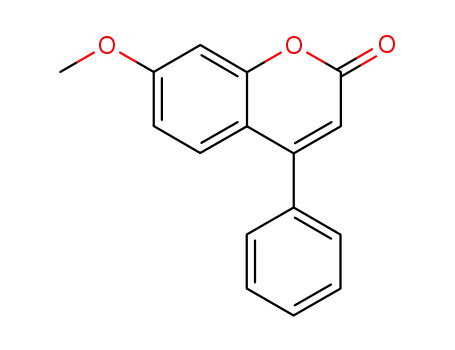
7-methoxy-4-phenyl-2H-chromen-2-one
-
35527-15-2
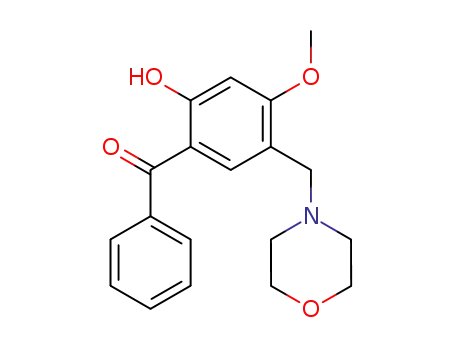
4-Methoxy-6-hydroxy-3-morpholinomethylbenzophenon
-
35527-14-1
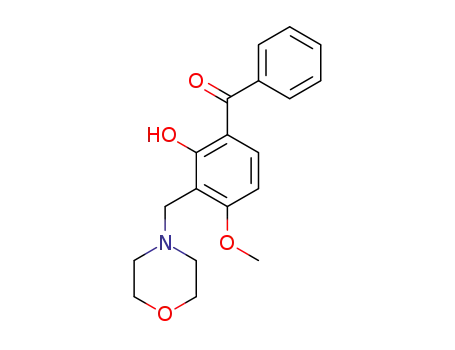
4-Methoxy-2-hydroxy-3-morpholinomethylbenzophenon
Relevant Products
-
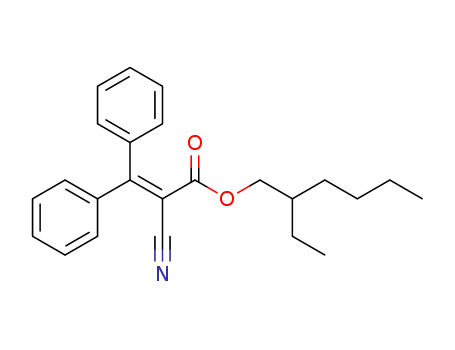
0ctocrylene
CAS:6197-30-4
-
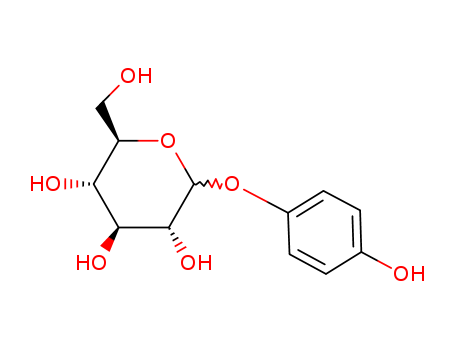
Alpha Arbutin
CAS:84380-01-8
-
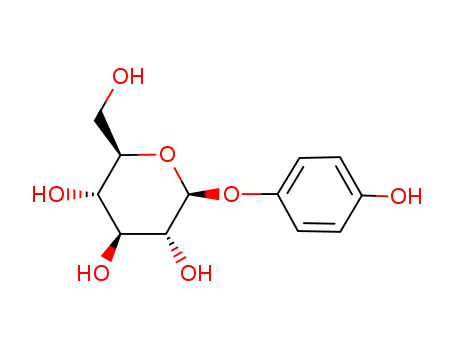
Beta Arbutin
CAS:497-76-7