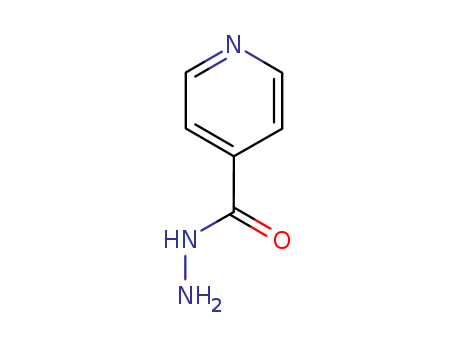
54-85-3
- Product Name:Isoniazid
- Molecular Formula:C6H7N3O
- Purity:99%
- Molecular Weight:137.141
Product Details
pd_meltingpoint:171-173 °C(lit.)
Appearance:white crystalline powder
Isoniazid Good Supplier In Bulk Supply High Purity 54-85-3
- Molecular Formula:C6H7N3O
- Molecular Weight:137.141
- Appearance/Colour:white crystalline powder
- Melting Point:171-173 °C(lit.)
- Refractive Index:1.577
- Boiling Point:251.97°C (rough estimate)
- PKA:pKa 2.00/3.60/10.8(H2O) (Uncertain)
- Flash Point:>250°C
- PSA:68.01000
- Density:1.244 g/cm3
- LogP:0.77630
Isoniazid(Cas 54-85-3) Usage
|
Indications |
Isoniazid (isonicotinic acid hydrazide, or INH) is the most active drug for the treatment of tuberculosis caused by susceptible strains. It is a synthetic agent with a structural similarity to that of pyridoxine. |
|
Manufacturing Process |
4 parts of 4-cyanopyridine in 12 parts of water were reacted with 4 parts of hydrazine hydrate in the presence of 0.08 part of sodium hydroxide at 100°C under reflux for 7 hours. The product, after filtration and evaporation to dryness, was crystallized from ethanol. The yield of isonicotinyl hydrazide amounted to 3.27 parts which is 62% of the theoretical. |
|
Therapeutic Function |
Antitubercular |
|
Biological Functions |
Its action is bactericidal against replicating organisms, but it appears to be only bacteriostatic at best against semidormant and dormant populations. After treatment with INH, M . tuberculosis loses its acid fastness, which may be interpreted as indicating that the drug interferes with cell wall development. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 20, p. 412, 1955 DOI: 10.1021/jo01122a002 |
|
Antimicrobial activity |
Susceptibility to isoniazid is virtually restricted to the M. tuberculosis complex (MIC 0.01–0.2 mg/L). It is highly bactericidal against actively replicating M. tuberculosis. Other mycobacteria are resistant, except for some strains of M. xenopi (MIC 0.2 mg/L) and a few strains of M. kansasii (MIC 1 mg/L). |
|
Acquired resistance |
Mutations in the katG gene, the inhA gene or its promoter region, and in the intergenic region of the oxyR–ahpC locus confer resistance to isoniazid. The relative proportions of such mutations vary geographically and are related to the distribution of the various lineages or superfamilies of M. tuberculosis. Isoniazid resistance is the commonest form of drug resistance worldwide and the great majority of strains resistant to another agent are also resistant to isoniazid. |
|
Air & Water Reactions |
Sensitive to air and light. Absorbs insignificant amounts of moisture at 77°F at relative humidities up to approximately 90%. Water soluble. Dust can be explosive when suspended in air at specific concentrations. |
|
Reactivity Profile |
Isoniazid is incompatible with chloral, aldehydes, iodine, hypochlorites and ferric salts. Isoniazid is also incompatible with oxidizers. Isoniazid may react with sugars and ketones. Isoniazid can react as a weak acid or a weak base. Isoniazid can be decomposed by oxidative and reductive reactions. |
|
Fire Hazard |
Isoniazid is combustible. |
|
Pharmaceutical Applications |
One of a number of nicotinamide analogs found to have antituberculosis activity, following the observation that nicotinamide inhibited the replication of M. tuberculosis. It is soluble in water. The dry powder is stable if protected from light. It is a prodrug requiring oxidative activation by KatG, a mycobacterial catalase–peroxidase enzyme. |
|
Biochem/physiol Actions |
Antibiotic for treatment of Mycobacterium tuberculosis, inhibits mycolic acid biosynthesis. Metabolized by hepatic N-acetyltransferase (NAT) and cytochrome P450 2E1 (CYP2E1) to form hepatotoxins. Selectively induces expression of CYP2E1. Reversibly inhibits CYP2C19 and CYP3A4 activities, and mechanistically inactivates CYP1A2, CYP2A6, CYP2C19 and CYP3A4 at clinically relevant concentrations. |
|
Mechanism of action |
Isoniazid is active against susceptible bacteria only when they are undergoing cell division. Susceptible bacteria may continue to undergo one or two divisions before multiplication is arrested. Isoniazid can inhibit the synthesis of mycolic acids, which are essential components of mycobacterial cell walls.The mycobacterial enzyme catalase– peroxidase KatG activates the administered isoniazid to its biologically active form.The target sites for the activated isoniazid action are acyl carrier protein AcpM and Kas A, a β-ketoaceyl carrier protein synthetase that blocks mycolic acid synthesis. Isoniazid exerts its lethal effects at the target sites by forming covalent complexes. |
|
Pharmacology |
Isoniazid is water soluble and is well absorbed when administered either orally or parenterally. Oral absorption is decreased by concurrent administration of aluminum-containing antacids. Isoniazid does not bind to serum proteins; it diffuses readily into all body fluids and cells, including the caseous tuberculous lesions. The drug is detectable in significant quantities in pleural and ascitic fluids, as well as in saliva and skin. The concentrations in the central nervous system (CNS) and cerebrospinal fluid are generally about 20% of plasma levels but may reach close to 100% in the presence of meningeal inflammation. Isoniazid is acetylated to acetyl isoniazid by N-acetyltransferase, an enzyme in liver, bowel, and kidney. Individuals who are genetically rapid acetylators will have a higher ratio of acetyl isoniazid to isoniazid than will slow acetylators. Rapid acetylators were once thought to be more prone to hepatotoxicity, but this is not proved. The slow or rapid acetylation of isoniazid is rarely important clinically, although slow inactivators tend to develop peripheral neuropathy more readily. Metabolites of isoniazid and small amounts of unaltered drug are excreted in the urine within 24 hours of administration. |
|
Pharmacokinetics |
Oral absorption: >95% Cmax 300 mg oral: 3–5 mg/L after 1–2 h Plasma half-life: 0.5–1.5 h (rapid acetylators): 2–4 h (slow acetylators) Volume of distribution: 0.6–0.8 L/kg Plasma protein binding: Very low Absorption and distribution Isoniazid is almost completely absorbed from the gut and is well distributed. Absorption is impaired by aluminum hydroxide. Therapeutic concentrations are achieved in sputum and CSF. It crosses the placenta and is found in breast milk. Metabolism Isoniazid is extensively metabolized to a variety of pharmacologically inactive derivatives, predominantly by acetylation. As a result of genetic polymorphism, patients are divisible into rapid and slow acetylators. About 50% of Caucasians and Blacks, but 80–90% of Chinese and Japanese, are rapid acetylators. Acetylation status does not affect the efficacy of daily administered therapy. The rate of acetylation is reduced in chronic renal failure. Excretion Nearly all the dose is excreted in the urine within 24 h, as unchanged drug and metabolic products. |
|
Side effects |
The incidence and severity of adverse reactions to isoniazid are related to dosage and duration of therapy. Isoniazid-induced hepatitis and peripheral neuropathy are two major untoward effects. |
|
Synthesis |
Isoniazid, isonicotinic acid hydrazide (34.1.1), is synthesized by reacting ethyl ester of isonicotinic acid with hydrazine. |
|
Veterinary Drugs and Treatments |
Isoniazid (INH) is sometimes used for chemoprophylaxis in small animals in households having a human with tuberculosis. It potentially can be used in combination with other antimycobacterial drugs to treat infections of M. bovis or M. tuberculosis in dogs or cats. But because of the public health risks, particularly in the face of increased populations of immunocompromised people, treatment of mycobacterial (M. bovis, M. tuberculosis) infections in domestic or captive animals is controversial. In addition, INH has a narrow therapeutic index and toxicity is a concern (see Adverse Effects). In humans, isoniazid (INH) is routinely used alone to treat latent tuberculosis infections (positive tuberculin skin test) and in combination with other antimycobacterial agents to treat active disease. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: increased risk of hepatotoxicity with rifampicin. Antiepileptics: metabolism of carbamazepine, ethosuximide and phenytoin inhibited (enhanced effect); also with carbamazepine, isoniazid hepatotoxicity possibly increased. |
|
Environmental Fate |
Isoniazid is a colorless, odorless, white crystalline powder that is slowly oxidized by exposure to air. It undergoes degradation upon prolonged exposure to light. Isoniazid has a solubility of 1 g per 8 ml water, 1 g per 50 ml ethanol, and it is slightly soluble in chloroform and very slightly soluble in ether. A 10% solution of isoniazid has a pH of 6.0–8.0. |
|
Metabolism |
Isoniazid is extensively metabolized to inactive metabolites. The major metabolite is N-acetylisoniazid. The enzyme responsible for acetylation, cytosolic N-acetyltransferase, is produced under genetic control in an inherited autosomal fashion. Individuals who possess high concentrations of the enzyme are referred to as rapid acetylators, whereas those with low concentrations are slow acetylators. This may result in a need to adjust the dosage for fast acetylators. The N-acetyltransferase is located primarily in the liver and small intestine. Other metabolites include isonicotinic acid, which is found in the urine as a glycine conjugate, and hydrazine. Isonicotinic acid also may result from hydrolysis of acetylisoniazid, but in this case, the second product of hydrolysis is acetylhydrazine. Acetylhydrazine is acetylated by N-acetyltransferase to the inactive diacetyl product. This reaction occurs more rapidly in rapid acetylators. The formation of acetylhydrazine is significant in that this compound has been associated with the hepatotoxicity, which may occur during INH therapy. |
|
Purification Methods |
Crystallise isoniazide from 95% EtOH and dry it in a vacuum. [Beilstein 22 III/IV 545, 22/2 V 219.] |
|
Toxicity evaluation |
Isoniazid causes toxicity by altering the metabolism of pyridoxine and creating a functional deficiency. Pyridoxine is needed for transamination, transketolization, decarboxylation, and biotransformation reactions. This occurs through three processes: (1) isoniazid metabolites form complexes with pyridoxine increasing its urinary excretion with increasing doses; (2) isoniazid metabolites disrupt the conversion of pyridoxine to its active form, pyridoxine-50-phosphokinase; and (3) metabolites directly inactivate pyridoxal-50-phosphate. Isoniazid-induced seizures are thought to be caused by the depletion of gamma-aminobutyric acid (GABA). GABA is the primary inhibitory neurotransmitter in the central nervous system that requires the cofactor pyridoxal-50-phosphate for its synthesis from glutamate. Prolonged seizures commonly result in plasma lactic acid accumulation that can lead to an anion gap metabolic acidosis. Isoniazid may worsen the severity of acidosis by inhibiting the production of nicotinamideadensosine dinucleotide (NAD), a cofactor necessary for the conversion of lactate to pyruvate. Long-term exposure to isoniazid therapy commonly causes peripheral neuropathy due to pyridoxine deficiency, and may induce pellagra, a niacin deficiency disorder. Niacin requires the cofactor pyridoxal-50- phosphate for its production from tryptophan. The exact mechanism of isoniazid-induced hepatotoxicity is unknown. However, it is thought to involve an idiopathic autoimmune mechanism or result from direct hepatic injury from isoniazid or its metabolites. The metabolite thought to be responsible is acetyl hydrazine, produced from isoniazid hydrolysis via cytochrome P450 (CYP)2E1. Persons with the CYP2E1c1/c1 genotype may be more susceptible to hepatotoxicity. The role acetylator status plays in hepatotoxicity continues to be debated, but it is currently thought that slow acetylators are at greater risk. Other risk factors include increasing age, chronic isoniazid overdose, comorbid conditions such as malnutrition, pregnancy, diabetes, HIV, renal dysfunction, hepatic dysfunction, alcoholism, and concomitant use of enzyme inducing drugs. Other enzymes inhibited by isoniazid include the cytochrome P450 mixed function oxidases, monoamine oxidase, glutamate decarboxylase, and histaminase. The consequences of these extensive enzymatic disturbances are mood elevation, decreased central nervous system GABA levels, depressed catecholamine synthesis, defects in glucose and fatty acid oxidation, and impaired metabolism of other drugs. Important drug interactions include those with carbamazepine, phenytoin, rifampin, theophylline, valproate, and warfarin. Isoniazid is also a weak monoamine oxidase inhibitor, and serotonin syndrome and tyramine reactions to foods causing flushing, tachycardia, and hypertension are reported. Isoniazid does cross the placenta and enters the fetal compartment; however, it has been determined to not be a human teratogen in studies. In acute toxicity, fetal deformities have been reported. |
|
Precautions |
High isoniazid plasma levels inhibit phenytoin metabolismand potentiate phenytoin toxicity when the twodrugs are coadministered. The serum concentrations ofphenytoin should be monitored, and the dose should beadjusted if necessary. |
|
Definition |
ChEBI: A carbohydrazide obtained by formal condensation between pyridine-4-carboxylic acid and hydrazine. |
|
Brand name |
Inh (Novartis); Nydrazid (Bristol-Myers Squibb); Nydrazid (Sandoz); Rimifon (Roche). |
|
General Description |
Odorless colorless or white crystals or white crystalline powder. Taste is slightly sweet at first and then bitter. pH (1% aqueous solution) 5.5-6.5. pH (5% aqueous solution) 6-8. |
InChI:InChI=1/C6H7N3O2/c7-9-11-6(10)5-1-3-8-4-2-5/h1-4,9H,7H2
54-85-3 Relevant articles
Solvent free biocatalytic synthesis of isoniazid from isonicotinamide using whole cell of Bacillus smithii strain IITR6b2
Agarwal, Shilpi,Gupta, Meenu,Choudhury, Bijan
, p. 67 - 73 (2013)
A biocatalytic route for the synthesis o...
Isoniazid
Brewer, Glenn A.
, p. 183 - 258 (1977)
-
-
Sycheva et al.
, (1972)
-
Design, docking, synthesis, and characterization of novel N'(2-phenoxyacetyl) nicotinohydrazide and N'(2-phenoxyacetyl)isonicotinohydrazide derivatives as anti-inflammatory and analgesic agents
Al-Ostoot, Fares Hezam,Khanum, Shaukath Ara,M, Pallavi H,Vivek, Hamse Kameshwar
, (2021/09/14)
Inflammation is the complex biological r...
Development of potent nanosized isatin-isonicotinohydrazide hybrid for management of Mycobacterium tuberculosis
Abdel-Aziz, Marwa M.,Abdelshafi, Nahla A.,Al-Rashood, Sara T.,Al-Zahraa Sayed, Fatma,Eissa, Noura G.,El Hassab, Mahmoud A.,Eldehna, Wagdy M.,Elkaeed, Eslam B.,Elsabahy, Mahmoud,Elsayed, Zainab M.,Fares, Mohamed
, (2021/12/20)
Inspired by the antitubercular activity ...
Pleuromutilin derivative with 1, 3, 4-oxadiazole side chain and preparation and application thereof
-
Paragraph 0055-0056; 0070; 0090; 0093; 0095; 0102, (2021/07/24)
The invention belongs to the field of me...
Discovery, synthesis and in combo studies of Schiff’s bases as promising dipeptidyl peptidase-IV inhibitors
Abu Khalaf, Reema,Al-Essa, Luay,Al-Shalabi, Eveen,Awad, Maha,Mefleh, Sara,Sabbah, Dima,Shabeeb, Ihsan
, (2021/09/25)
Abstract: Diabetes mellitus is a main gl...
54-85-3 Process route
-
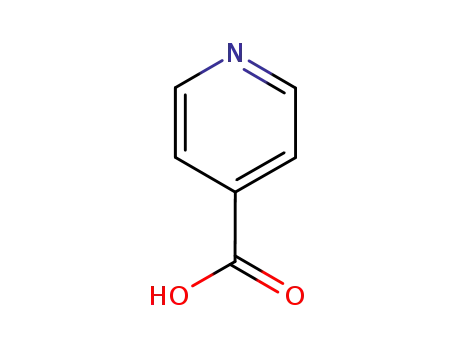
-
55-22-1
pyridine-4-carboxylic acid

-
-
302-01-2,78206-91-4,119775-10-9,75013-58-0
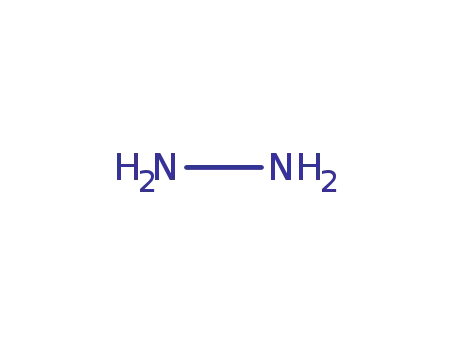
hydrazine

-
-
54-85-3,7640-37-1
isoniazid

-
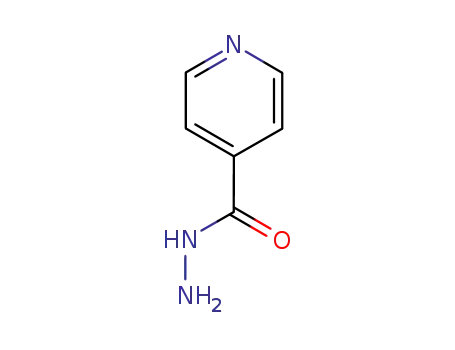
-
4329-75-3
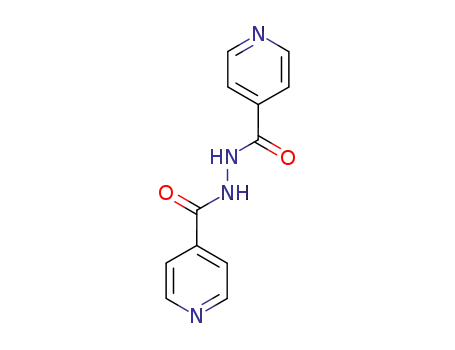
N,N'-bis(isonicotinoyl)hydrazine

-
-
38634-05-8
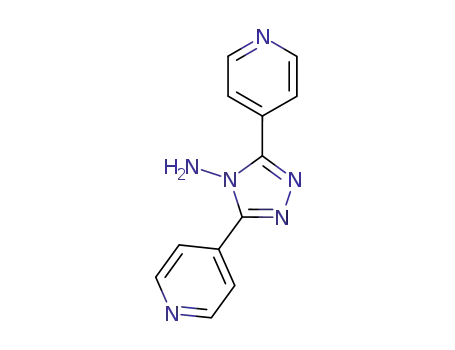
3,5-di(pyridin-4-yl)-4H-1,2,4-triazol-4-amine
| Conditions | Yield |
|---|---|
|
at 130 ℃;
|
-
![{3-hydroxy-4-[(E)-(isonicotinoylhydrazono)methyl]-2-methylpyridin-5-yl}methylphosphate](/upload/2024/7/b0a30a82-91fd-4a40-b0ea-7d516610cbee.png)
-
53-91-8
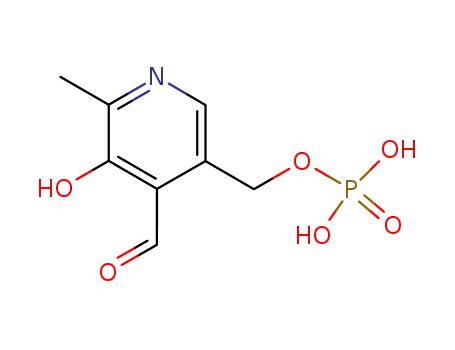
{3-hydroxy-4-[(E)-(isonicotinoylhydrazono)methyl]-2-methylpyridin-5-yl}methylphosphate

-

-
54-85-3,7640-37-1
isoniazid

-
-
54-47-7
pyridoxal 5'-phosphate
| Conditions | Yield |
|---|---|
|
at 25 ℃;
Equilibrium constant;
Rate constant;
depending on pH;
|
54-85-3 Upstream products
-
100-48-1
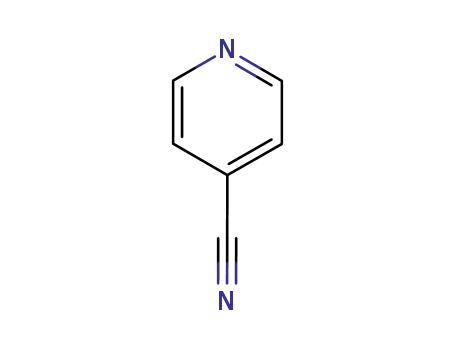
pyridine-4-carbonitrile
-
55-22-1

pyridine-4-carboxylic acid
-
1570-45-2
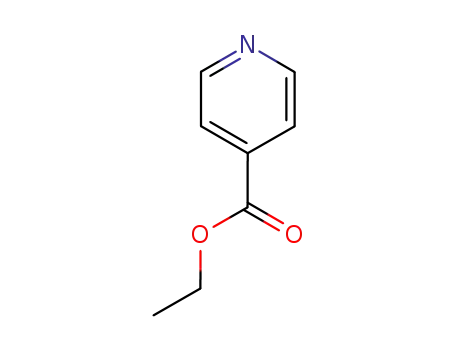
isonicotinic acid ethylester
-
1453-82-3
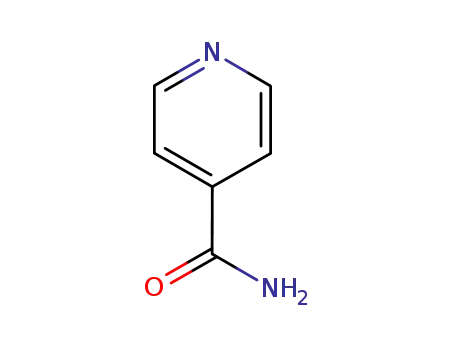
isonicotinamide
54-85-3 Downstream products
-
103033-32-5
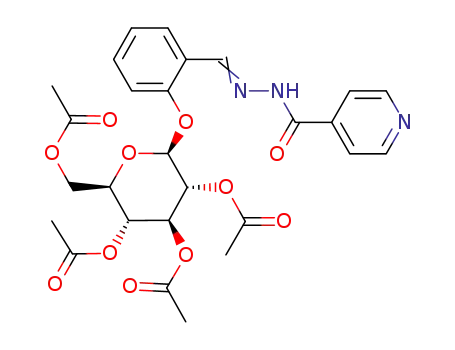
isonicotinic acid-[2-(tetra-O-acetyl-β-D-glucopyranosyloxy)-benzylidenehydrazide]
-
57654-36-1
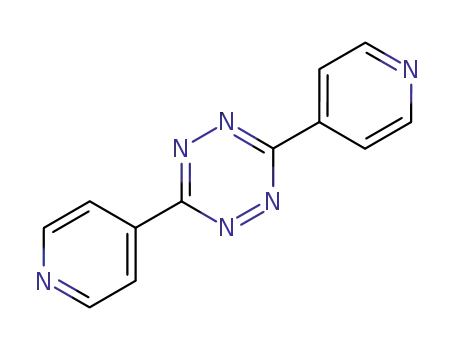
3,6-di(pyridin-4-yl)-1,2,4,5-tetrazine
-
15017-32-0
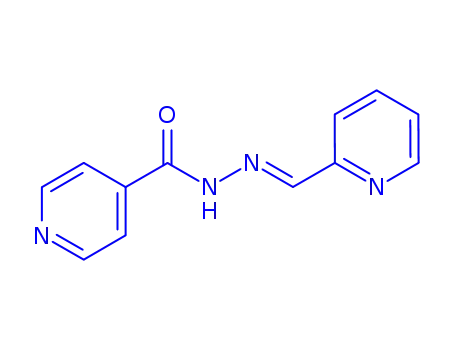
2-pyridylcarboxaldehyde isonicotinoyl hydrazone
-
13025-99-5
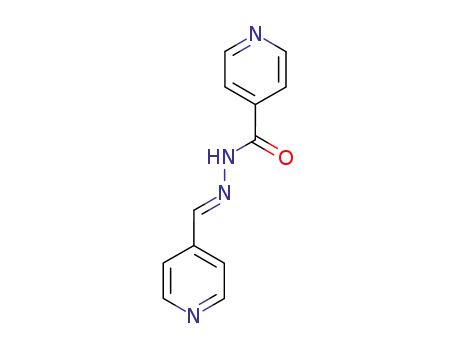
(E)-N′-(pyridin-4-ylmethylene) isonicotinohydrazide
Relevant Products
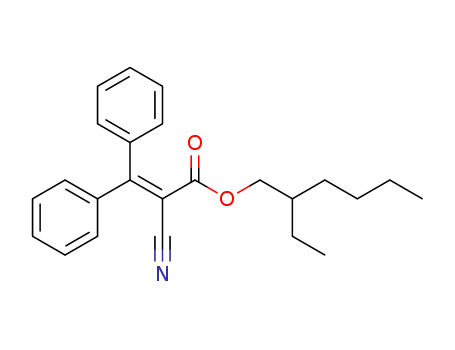
-
0ctocrylene
CAS:6197-30-4
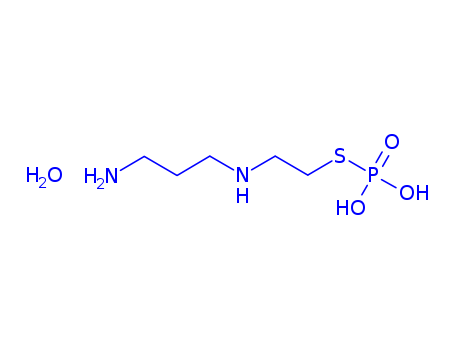
-
Amifostine trihydrate
CAS:112901-68-5
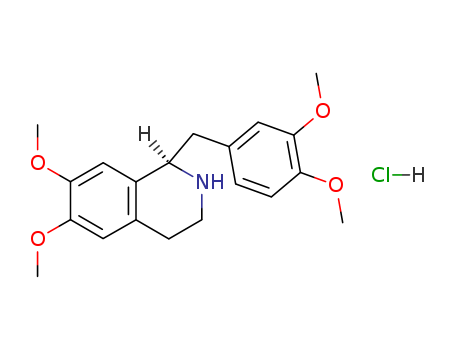
-
R-Tetrahydropapaverine
CAS:54417-53-7