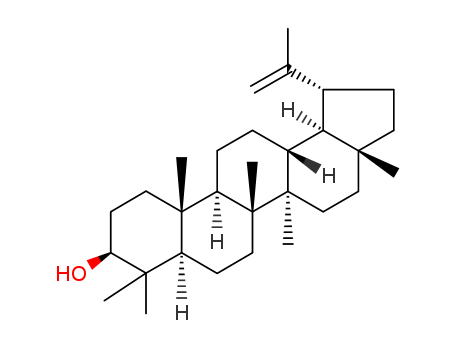
Product Details
pd_meltingpoint:215-216oC
Appearance:white powder
Purity 99% Min Lupeol 545-47-1 Spot Supply with Safe Transportation
- Molecular Formula:C30H50O
- Molecular Weight:426.726
- Appearance/Colour:white powder
- Vapor Pressure:1.41E-11mmHg at 25°C
- Melting Point:215-216oC
- Refractive Index:1.515
- Boiling Point:488.1 °C at 760 mmHg
- PKA:15.19±0.70(Predicted)
- Flash Point:216.9 °C
- PSA:20.23000
- Density:0.978 g/cm3
- LogP:8.02480
Lupeol(Cas 545-47-1) Usage
|
Definition |
ChEBI: A pentacyclic triterpenoid that is lupane in which the hydrogen at the 3beta position is substituted by a hydroxy group. It occurs in the skin of lupin seeds, as well as in the latex of fig trees and of rubber plants. It is also found in m ny edible fruits and vegetables. |
|
General Description |
Lupeol is a novel anti-cancer and anti-inflammatory dietary triterpene. They help in stabilizing phospholipid bilayers in plant cell membranes. In general, this group contains 28-29 carbons along with carbon-carbon double bonds, usually one in the sterol nucleus and at times a second in the alkyl side chain. |
InChI:InChI=1/C30H50O/c1-19(2)20-11-14-27(5)17-18-29(7)21(25(20)27)9-10-23-28(6)15-13-24(31)26(3,4)22(28)12-16-30(23,29)8/h20-25,31H,1,9-18H2,2-8H3/t20-,21+,22-,23+,24-,25+,27+,28-,29+,30+/m0/s1
545-47-1 Relevant articles
Pentacyclic triterpenes and naphthoquinones from Euclea divinorum
Mebe, Paul P.,Cordell, Geoffrey A.,Pezzuto, John M.
, p. 311 - 313 (1998)
Phytochemical studies on Euclea divinoru...
TRITERPENOIDS OF AMSONIA GRANDIFLORA
Wahyuono, Subagus,Hoffmann, Joseph J.,Jolad, Shivanand D.,Dentali, Steven J.
, p. 1213 (1987)
Stems and leaves of Amsonia grandiflora ...
Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark
Weigenand, Oliver,Hussein, Ahmed A.,Lall, Namrita,Meyer, Jacobus J. M.
, p. 1936 - 1938 (2004)
Phytochemical studies of an ethanolic ex...
Synthesis of Lupeol from Betulin
Garifullina,Myasoedova, Yu. V.,Ishmuratova
, p. 765 - 767 (2019/08/07)
-
Practical Synthesis of α-Amyrin, β-Amyrin, and Lupeol: The Potential Natural Inhibitors of Human Oxidosqualene Cyclase
Chen, Dongyin,Xu, Fengguo,Zhang, Pu,Deng, Jie,Sun, Hongbin,Wen, Xiaoan,Liu, Jun
, (2017/10/20)
A practical synthesis of α-amyrin (1), β...
And reclaims to clean rough synthesis method
-
Paragraph 0036-0041; 0053-0055, (2017/08/25)
The invention belongs to the research fi...
Lupeol-3-O-decanoate, a new triterpene ester from Cadaba farinosa Forssk. growing in Saudi Arabia
Al-Musayeib, Nawal M.,Mohamed, Gamal A.,Ibrahim, Sabrin R. M.,Ross, Samir A.
, p. 5297 - 5302 (2013/12/04)
A new triterpene ester (1) together with...
545-47-1 Process route
-
![Pentadecanoic acid (1R,3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-1-isopropenyl-3a,5a,5b,8,8,11a-hexamethyl-icosahydro-cyclopenta[a]chrysen-9-yl ester](/upload/2024/7/24782d4c-bb2c-4a6c-aa92-a6cc455cbc04.png)
-
Pentadecanoic acid (1R,3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-1-isopropenyl-3a,5a,5b,8,8,11a-hexamethyl-icosahydro-cyclopenta[a]chrysen-9-yl ester

-
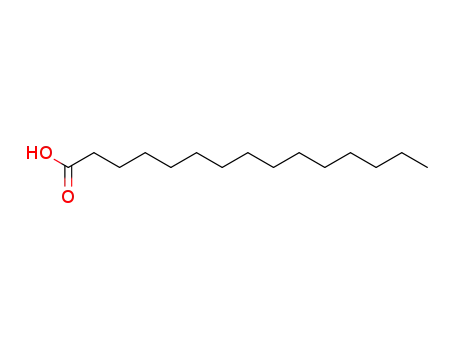
- 1002-84-2
palmitic acid

-
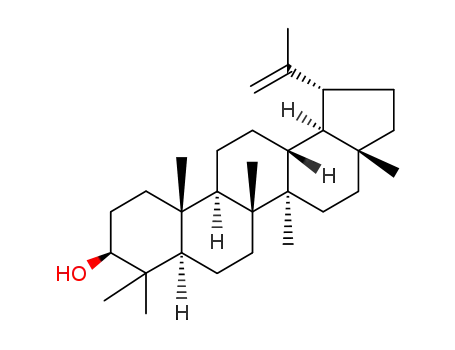
- 545-47-1
lupenol
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In chloroform; for 3h; Heating;
|
-
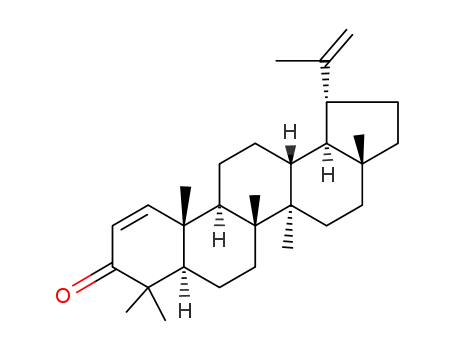
- 6610-55-5
Glochidone

-

- 545-47-1
lupenol

-
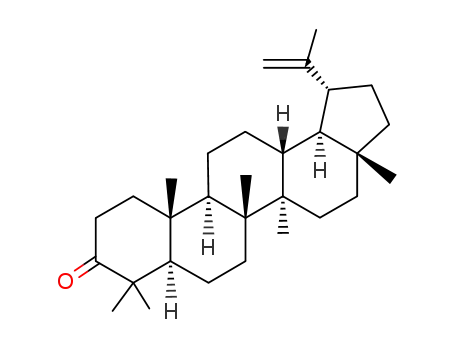
- 1617-70-5
lupenone

-
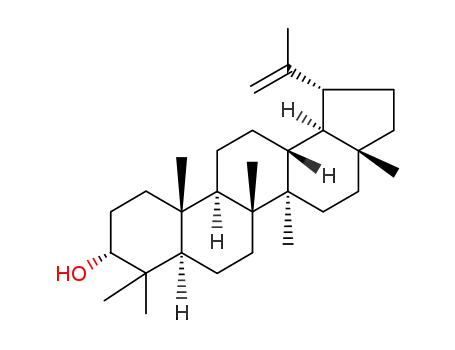
- 33869-84-0,55870-42-3,101627-40-1,107596-77-0,545-47-1,4439-99-0
3-epilupeol
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In diethylene glycol; for 2h; Heating;
|
40% 15% 8% |
545-47-1 Upstream products
-
1617-70-5

lupenone
-
555-31-7
aluminum isopropoxide
-
67-63-0
isopropyl alcohol
-
71-43-2
benzene
545-47-1 Downstream products
-
3186-86-5
3β-Lupanol
-
3186-86-5
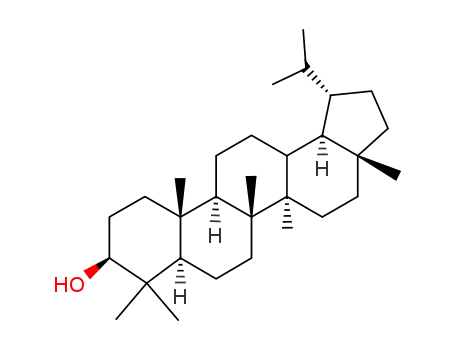
3β-Hydroxy-28-lupane
-
1617-68-1
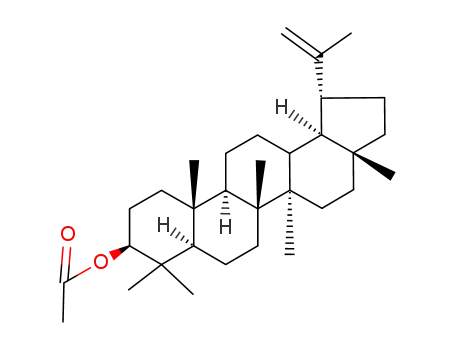
lupeol acetate
-
3186-72-9
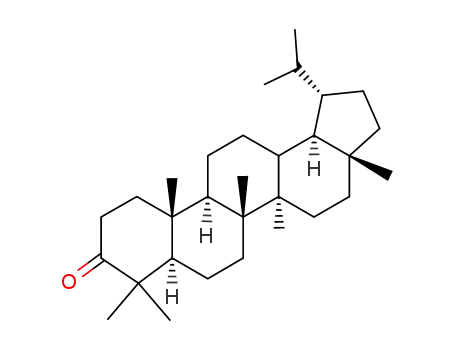
3-Oxolupane
Relevant Products
-
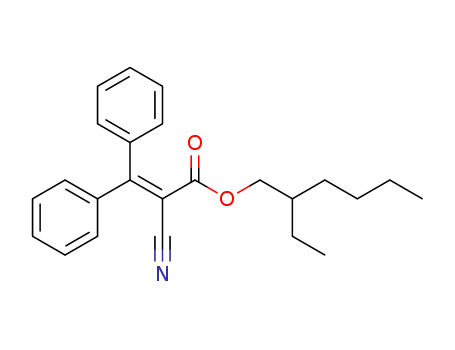
0ctocrylene
CAS:6197-30-4
-
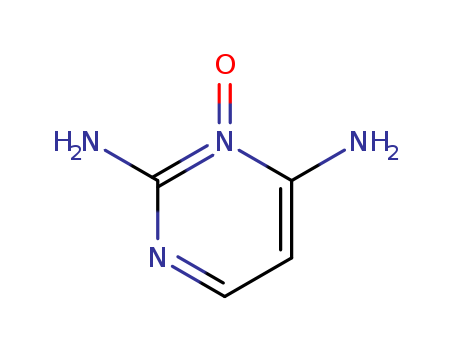
Diaminopyrimidine Oxide
CAS:74638-76-9
-
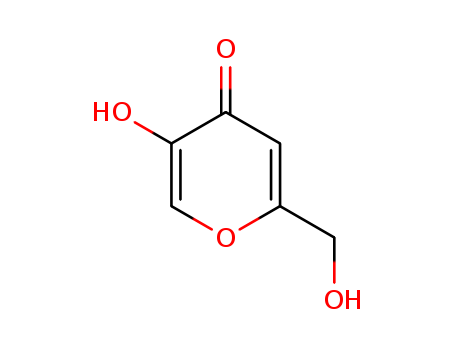
Kojic Acid
CAS:501-30-4