10597-60-1
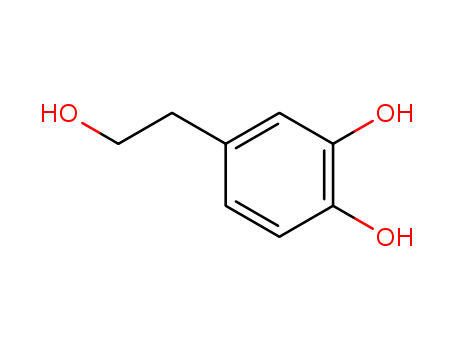
- Product Name:Hydroxytyrosol
- Molecular Formula:C8H10O3
- Purity:99%
- Molecular Weight:154.166
Product Details
Appearance:Yellow green powder
Manufacturer Supply Best Quality Hydroxytyrosol 10597-60-1 with Efficient Transportation
- Molecular Formula:C8H10O3
- Molecular Weight:154.166
- Appearance/Colour:Yellow green powder
- Vapor Pressure:1.15E-05mmHg at 25°C
- Refractive Index:1.622
- Boiling Point:355.4 °C at 760 mmHg
- PKA:9.72±0.10(Predicted)
- Flash Point:182.6 °C
- PSA:60.69000
- Density:1.321 g/cm3
- LogP:0.63260
3,4-Dihydroxyphenylethanol(Cas 10597-60-1) Usage
|
Characteristics |
Hydroxytyrosol displays much more effective antioxidant characteristics, such as the scavenging of free radicals, breaking peroxidative chain reactions, preventing lipid peroxidation, inhibiting hypochlorous acid derived radicals, and so on, compared with other phenolic compounds in olive oil. It could be used in the dermocosmetic industry for the creation of products for protecting the skin from oxidative stress or used as a preservative in the food technology. |
|
Biochem/physiol Actions |
Metabolite of oleuropein. Antioxidant. Inhibits the rate of cancer cell proliferation and induces cancer cell apoptosis. |
|
Source |
Hydroxytyrosol is also known as 2-(3,4-dihydroxyphenyl)-ethanol (3,4-DHPEA) and as DOPET. Hydroxytyrosol is mainly found in olive oil as secoiridoid derivatives, as acetate and in free form. Both hydroxytyrosol and its derivatives arise from oleuropein (hydroxytyro- sol esterified with elenolic acid), present in olives during the extraction of olive oil.Wine has proven to be another important source of hydroxytyrosol in the Mediterranean diet, and is formed in wine from tyrosol during alcoholic fermentation. Hydroxytyrosol was firstly found in Italian wines by Di Tommaso et al., and later in other Italian and Greek wines. Some authors describe a higher concentration in red wines (3.66-4.20 mg/L-1) than in white wines (1.72-1.92mg/L-1). Finally, Minuti et al. obtained hydroxytyrosol concen- trations between 1.8 and 3.1 mg L-1 in red wine. Thus, scientific literature shows that wine is an important source of hydroxytyrosol in the diet, along with olive oil. |
|
Definition |
ChEBI: Hydroxytyrosol is a member of the class of catechols that is benzene-1,2-diol substituted by a 2-hydroxyethyl group at position 4. Isolated from Olea europaea, it exhibits antioxidant and antineoplastic activities. It has a role as a metabolite, an antioxidant and an antineoplastic agent. It is a member of catechols and a primary alcohol. It derives from a 2-(4-hydroxyphenyl)ethanol. |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products. Other release to the environment of this substance is likely to occur from: indoor use in close systems with minimal release (e.g. cooling liquids in refrigerators, oil-based electric heaters). |
InChI:InChI=1/C8H10O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,9-11H,3-4H2
10597-60-1 Relevant articles
Olea europaea chemicals repellent to Dacus oleae females
Scalzo,Scarpati,Verzegnassi,Vita
, p. 1813 - 1823 (1994)
-
Synthesis of hydroxytyrosol, 2-hydroxyphenylacetic acid, and 3-hydroxyphenylacetic acid by differential conversion of tyrosol isomers using Serratia marcescens strain
Allouche, Noureddine,Sayadi, Sami
, p. 6525 - 6530 (2005)
We investigated to develop an effective ...
Antioxidant activity of olive phenols: Mechanistic investigation and characterization of oxidation products by mass spectrometry
Roche, Marjolaine,Dufour, Claire,Mora, Nathalie,Dangles, Olivier
, p. 423 - 430 (2005)
In this work, the antioxidant activity o...
Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection
Grasso, Salvatore,Siracusa, Laura,Spatafora, Carmela,Renis, Marcella,Tringali, Corrado
, p. 137 - 152 (2007)
The olive oil phenol hydroxytyrosol (3),...
Synthesis of tritium-labeled hydroxytyrosol, a phenolic compound found in olive oil
Tuck, Kellie L.,Tan, Hai-Wei,Hayball, Peter J.
, p. 4087 - 4090 (2000)
(3,4-Dihydroxyphenyl)ethanol, commonly k...
Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection
Mateos, Raquel,Espartero, Jose Luis,Trujillo, Mariana,Rios,Leon-Camacho, Manuel,Alcudia, Felipe,Cert, Arturo
, p. 2185 - 2192 (2001)
A simple analytical method for the quant...
A highly convenient synthesis of hydroxytyrosol and its recovery from agricultural waste waters
Capasso, Renato,Evidente, Antonio,Avolio, Salvatore,Solla, Francesco
, p. 1745 - 1748 (1999)
Hydroxytyrosol, a polyphenol with very i...
Oxidative cleavage of 1-aryl-isochroman derivatives using the: Trametes villosa laccase/1-hydroxybenzotriazole system
Bernini, Roberta,Crisante, Fernanda,D'Acunzo, Francesca,Gentili, Patrizia,Ussia, Emanuele
, p. 3314 - 3322 (2016)
The oxidative cleavage of the dihydropyr...
Determination of hydroxytyrosol in plasma by HPLC
Ruiz-Gutierrez,Juan,Cert,Planas
, p. 4458 - 4461 (2000)
Hydroxytyrosol (2-(3,4-dihydroxyphenyl)e...
Characterization of Type IV Carboxylate Reductases (CARs) for Whole Cell-Mediated Preparation of 3-Hydroxytyrosol
Horvat, Melissa,Fritsche, Susanne,Kourist, Robert,Winkler, Margit
, p. 4171 - 4181 (2019)
Fragrance and flavor industries could no...
High-yielding preparation of a stable precursor of hydroxytyrosol by total synthesis and from the natural glycoside oleuropein
Gambacorta, Augusto,Tofani, Daniela,Bernini, Roberta,Migliorini, Antonella
, p. 3386 - 3391 (2007)
The unprecedented acetonide of the antio...
Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions
Medina,Lois,Alcantara,Lucas,Morales
, p. 9773 - 9779 (2009)
The effect of lipophilization of the ant...
HYDROXYCINNAMIC ACID ESTERS OF PHENETHYLALCOHOL GLYCOSIDES FROM REHMANNIA GLUTINOSA VAR. PURPUREA
Sasaki, Hiroshi,Nishimura, Hiroaki,Chin (Chen Zhengxiong), Masao,Mitsuhashi, Hiroshi
, p. 875 - 880 (1989)
Five new hydroxycinnamic acid esters of ...
Main antimicrobial compounds in table olives
Medina, Eduardo,Brenes, Manuel,Romero, Concepcion,Garcia, Aranzazu,De Castro, Antonio
, p. 9817 - 9823 (2007)
The inhibitors involved in the lactic ac...
Determination of Synthetic Hydroxytyrosol in Rat Plasma by GC-MS
Bai, Chen,Yan, Xiaojun,Takenaka, Makiko,Sekiya, Keizo,Nagata, Tadahiro
, p. 3998 - 4001 (1998)
2-(3,4-Dihydroxyphenyl)ethanol (DPE), th...
Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst
Espin, Juan Carlos,Soler-Rivas, Cristina,Cantos, Emma,Tomas-Barberan, Francisco A.,Wichers, Harry J.
, p. 1187 - 1193 (2001)
Hydroxytyrosol (HTyr), a natural ortho-d...
Production of hydroxytyrosol from hydroxylation of tyrosol by Rhodococcus pyridinivorans 3HYL DSM109178
Anissi, Jaouad,Sendide, Khalid,Ouardaoui, Abdelkrim,Benlemlih, Mohammed,El Hassouni, Mohammed
, p. 418 - 428 (2021)
Hydroxytyrosol (4-(2-hydroxyethyl)-1,2-b...
Verbascoside from Verbascum phlomoides
Gvazava,Kikoladze
, p. 710 - 711 (2007)
-
Whole-cell carboxylate reduction for the synthesis of 3-hydroxytyrosol
Napora-Wijata, Kamila,Robins, Karen,Osorio-Lozada, Antonio,Winkler, Margit
, p. 1089 - 1095 (2014)
3-Hydroxytyrosol (3-HT) is a phenolic an...
Synthesis and Antioxidant Activity of Hydroxytyrosol Alkyl-Carbonate Derivatives
Fernandez-Pastor, Ignacio,Fernandez-Hernandez, Antonia,Rivas, Francisco,Martinez, Antonio,Garcia-Granados, Andres,Parra, Andres
, p. 1737 - 1745 (2016)
Three procedures have been investigated ...
Acteoside as the analgesic principle of Cedron (Lippia triphylla), a Peruvian medicinal plant
Nakamura, Tomonori,Okuyama, Emi,Tsukada, Atsushi,Yamazaki, Mikio,Satake, Motoyoshi,Nishibe, Sansei,Deyama, Takeshi,Moriya, Akira,Maruno, Masao,Nishimura, Hiroaki
, p. 499 - 504 (1997)
Acteoside (verbascoside) was isolated as...
Potential antitumor agents from Lantana camara: Structures of flavonoid-, and phenylpropanoid glycosides
Mahato,Sahu,Roy,Sharma
, p. 9439 - 9446 (1994)
Besides the known glycosides, verbascosi...
Oxidative chemistry of the natural antioxidant hydroxytyrosol: Hydrogen peroxide-dependent hydroxylation and hydroxyquinone/o-quinone coupling pathways
De Lucia, Maria,Panzella, Lucia,Pezzella, Alessandro,Napolitano, Alessandra,D'Ischia, Marco
, p. 1273 - 1278 (2006)
Oxidation of the natural antioxidant hyd...
Two new phenylethanoid glycosides from Ginkgo biloba leaves and their tyrosinase inhibitory activities
Fei, Yingying,Li, Junping,Liu, Anqi,Liu, Wanrong,Niu, Haoying,Shu, Penghua,Wei, Xialan,Xiao, Fugang,Xu, Zhihong,Zhang, Lingxiang
, (2020)
Two undescribed phenylethanoid glycoside...
Novel approach to the detection and quantification of phenolic compounds in olive oil based on 31P nuclear magnetic resonance spectroscopy
Christophoridou, Stella,Dais, Photis
, p. 656 - 664 (2006)
31P NMR spectroscopy has been employed t...
Thiols Act as Methyl Traps in the Biocatalytic Demethylation of Guaiacol Derivatives
Grimm, Christopher,Kroutil, Wolfgang,Pompei, Simona,Schiller, Christine,Schober, Lukas
, p. 16906 - 16910 (2021)
Demethylating methyl phenyl ethers is ch...
A two-step process for the synthesis of hydroxytyrosol
Ziosi, Paolo,Paolucci, Claudio,Santarelli, Francesco,Tabanelli, Tommaso,Passeri, Sauro,Cavani, Fabrizio,Righi, Paolo
, p. 2202 - 2210 (2018)
A new process for the synthesis of hydro...
Expedient synthesis of hydroxytyrosol and its esters
Bovicelli, Paolo,Antonioletti, Roberto,Mancini, Silvia,Causio, Stefano,Borioni, Giorgio,Ammendola, Sergio,Barontini, Maurizio
, p. 4245 - 4252 (2007)
An efficient and friendly method for obt...
Regioselectivity of Cobalamin-Dependent Methyltransferase Can Be Tuned by Reaction Conditions and Substrate
Pompei, Simona,Grimm, Christopher,Farnberger, Judith E.,Schober, Lukas,Kroutil, Wolfgang
, p. 5977 - 5983 (2020)
Regioselective reactions represent a sig...
Improving process conditions of hydroxytyrosol synthesis by toluene-4-monooxygenase
Brouk, Moran,Fishman, Ayelet
, p. 121 - 127 (2012)
Toluene-4-monooxygenase from Pseudomonas...
Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity
Jabeen, Bushra,Riaz, Naheed,Saleem, Muhammad,Naveed, Muhammad Akram,Ashraf, Muhammad,Alam, Umber,Rafiq, Hafiza Mehwish,Tareen, Rasool Bakhsh,Jabbar, Abdul
, p. 443 - 448 (2013)
Stewartiiside (1), a phenylethanoid glyc...
Synthesis and biological evaluation of caffeic acid 3,4-dihydroxyphenethyl ester
Zhang, Zhizhen,Xiao, Binghua,Chen, Qi,Lian, Xiao-Yuan
, p. 252 - 254 (2010)
A high-yield synthesis of caffeic acid 3...
Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation
Maiuri, Maria Chiara,De Stefano, Daniela,Di Meglio, Paola,Irace, Carlo,Savarese, Maria,Sacchi, Raffaele,Cinelli, Maria Pia,Carnuccio, Rosa
, p. 457 - 465 (2005)
We investigated the effect of hydroxytyr...
Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme
Torrens-Spence, Michael P.,Gillaspy, Glenda,Zhao, Bingyu,Harich, Kim,White, Robert H.,Li, Jianyong
, p. 211 - 216 (2012)
Plant aromatic amino acid decarboxylases...
Production of high hydroxytyrosol yields via tyrosol conversion by Pseudomonas aeruginosa immobilized resting cells
Bouallagui, Zouhaier,Sayadi, Sami
, p. 9906 - 9911 (2006)
An immobilized whole cell system was suc...
Synthesis process of hydroxytyrosol and intermediate thereof
-
, (2022/03/27)
The invention discloses a synthesis proc...
Transaminase-Mediated Amine Borrowing via Shuttle Biocatalysis
O'Reilly, Elaine,O'Sullivan, Rachel,Ryan, James,Taday, Freya
supporting information, (2022/01/04)
Shuttle catalysis has emerged as a usefu...
Rapid biosynthesis of phenolic glycosides and their derivatives from biomass-derived hydroxycinnamates
Zhao, Mingtao,Hong, Xulin,Abdullah,Yao, Ruilian,Xiao, Yi
supporting information, p. 838 - 847 (2021/02/09)
Biomass-derived hydroxycinnamates (mainl...
10597-60-1 Process route
-
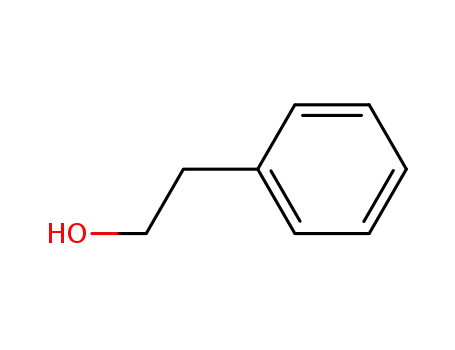
- 60-12-8
2-phenylethanol

-
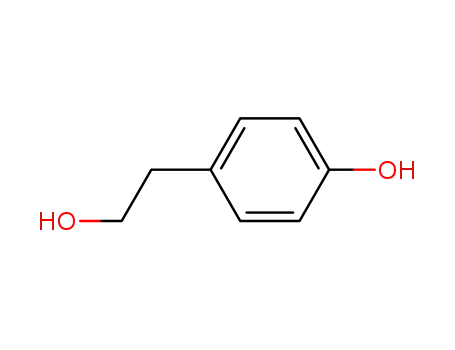
- 501-94-0
p-hydroxyphenethyl alcohol

-
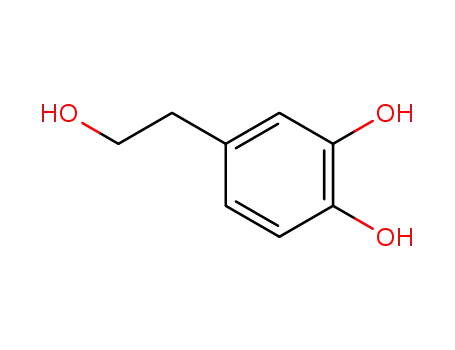
- 10597-60-1
hydroxytyrosol

-
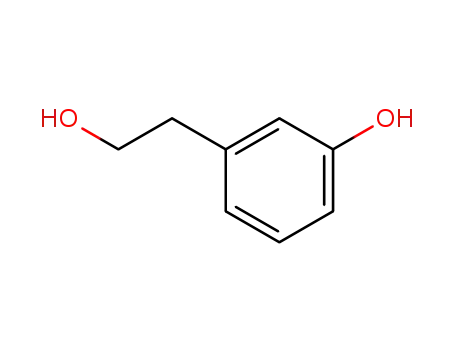
- 13398-94-2
2-(3-hydroxyphenyl)ethanol
| Conditions | Yield |
|---|---|
|
With Escherichia coli TG1 expressing Pseudomonas mendocina KR1 toluene-4-monooxygenase I100A/E214G/D285Q variant; In aq. phosphate buffer; ethanol; at 30 ℃; pH=7; Enzymatic reaction;
|
-

- 60-12-8
2-phenylethanol

-

- 501-94-0
p-hydroxyphenethyl alcohol

-

- 10597-60-1
hydroxytyrosol

-
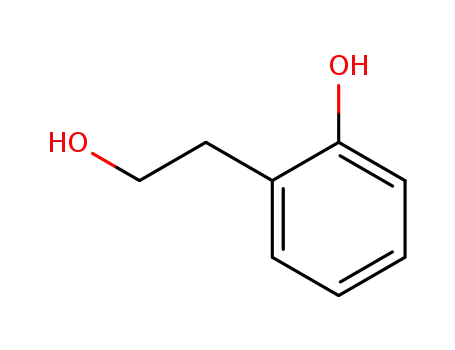
- 7768-28-7
2-(2-hydroxyphenyl)ethanol

-

- 13398-94-2
2-(3-hydroxyphenyl)ethanol
| Conditions | Yield |
|---|---|
|
With dihydrogen peroxide; In water; at 20 ℃; for 8h; pH=Ca. 2.8; Concentration; Time; chemoselective reaction; Catalytic behavior; Green chemistry;
|
10597-60-1 Upstream products
-
67-56-1
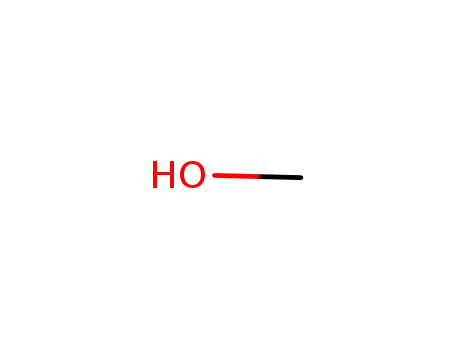
methanol
-
94492-24-7
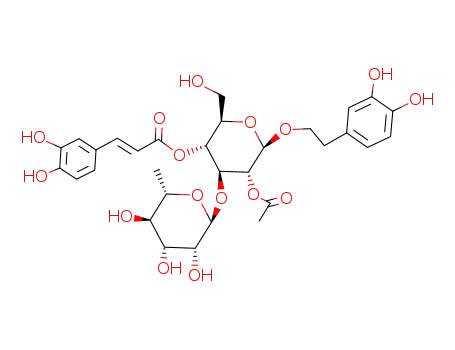
2′-acetylacteoside
-
112516-04-8
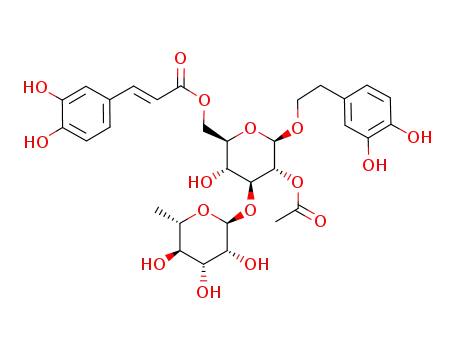
2'-acetylisoacteoside
-
112516-05-9
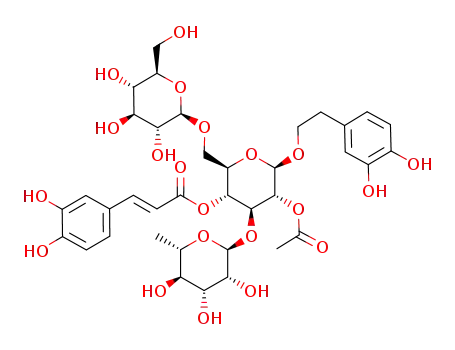
2-(3,4-dihydroxyphenyl)-ethyl O-α-L-rhamnopyranosyl-(1->3)-O-[β-D-clucopyranosyl-(1->6)]-2-O-acetyl-4-O-[(E)-caffeoyl]-β-D-glucopyranoside
10597-60-1 Downstream products
-
7417-21-2
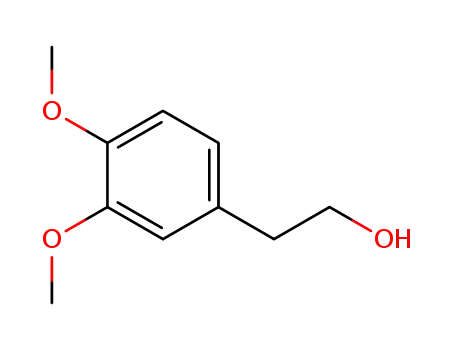
2-(3,4-dimethoxyphenyl)ethyl alcohol
-
86214-97-3
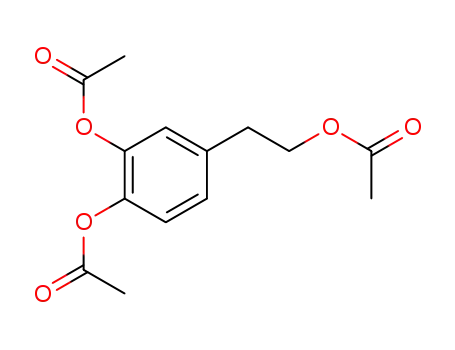
2-(3,4-diacetoxyphenyl)-ethyl acetate
Relevant Products
-
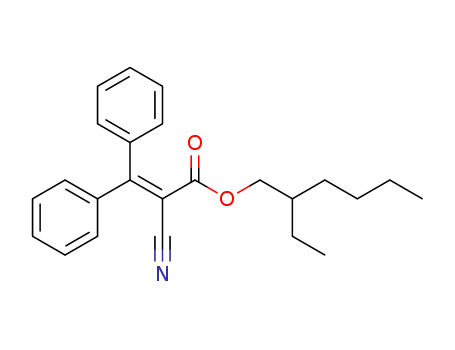
0ctocrylene
CAS:6197-30-4
-
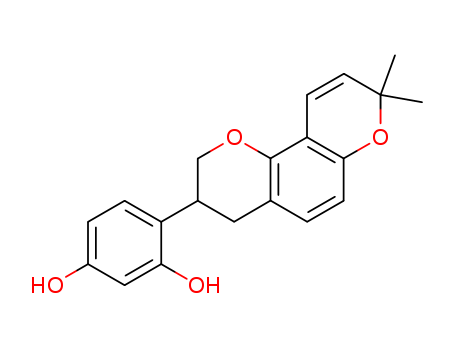
Glabridin
CAS:59870-68-7
-
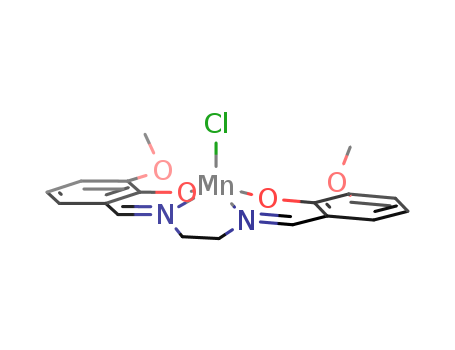
Ethyleneiminomethylguaiacol manganese chloride
CAS:81065-76-1