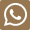
57-88-5
- Product Name:Cholesterol
- Molecular Formula:C27H46O
- Purity:99%
- Molecular Weight:386.662
Product Details
pd_meltingpoint:148-150 °C
Appearance:White to faintly yellow cryst. powder
Buy reliable Quality Cholesterol 57-88-5 raw material with Honest Price
- Molecular Formula:C27H46O
- Molecular Weight:386.662
- Appearance/Colour:White to faintly yellow cryst. powder
- Vapor Pressure:2.95E-11mmHg at 25°C
- Melting Point:148-150 °C
- Refractive Index:1.525
- Boiling Point:480.648 °C at 760 mmHg
- PKA:15.03±0.70(Predicted)
- Flash Point:209.291 °C
- PSA:20.23000
- Density:0.988 g/cm3
- LogP:7.38870
Cholesterol(Cas 57-88-5) Usage
|
Outline |
Cholesterol is a kind of derivatives of cyclopentane multiple hydrogen phenanthrenes and is an important component of various parts of membrane phase structure and myelin cells of human being. For the normal person with weight of 70 kg, the body contains about 140 grams of cholesterol. Since the early 18th century, people had already discovered cholesterol from gallstones. At 1816, Chemist Marshall named this kind of lipid-property substance as cholesterol. Cholesterol is widely found and distributed in animal bodies, and is especially most abundant in the brain and nerve tissue. It also has high content in the kidney, spleen, skin, liver and bile. The solubility of cholesterol is similar to that of the fat which is insoluble in water, but easily soluble in ether, chloroform and some other solvents. Cholesterol is closely related to the body tissues, bile acids and hormones. It is an indispensable substance of animal tissue cells, which is not only involved in the formation of cell membranes, but also as the precursor for the synthesis of bile acids, steroid hormones and vitamin D3. There are two sources of cholesterol with exogenous sources of cholesterol coming from dietary and endogenous sources coming from the body's own endogenous synthesis. The increased exogenous cholesterol can cause feedback inhibition of endogenous cholesterol synthesis. Fat in your diet can boost the cholesterol absorption. Cholesterol can be metabolized into bile acids or steroids. The absorption of cholesterol also depends on the cholesterol intake with high intake causing reduction of the percentage of absorption. The absorption percentage of cholesterol in people at high levels of intake is less than 10% with the rest part excreted through the feces. Dietary cholesterol is absorbed in the form of chylomicrons into the bloodstream. Because cholesterol can’t be dissolved in water and transported in the form of binding with lipoproteins in the blood. The main physiological function of cholesterol participating in forming cell membranes, myelin, brain, and can be further converted to bile acids and steroid hormones. There are two major lipoproteins which are involved in cholesterol transport: the low density lipoprotein (LDL) and high density lipoprotein (HDL). The former can transport cholesterol from the liver to the cells of whole body tissues, while the later one transports cholesterol from tissue cells back into the liver. The cholesterol level in the blood can reflect the overall metabolism condition of cholesterol. The total cholesterol content in the blood plasma of normal adult of empty stomach is about 2.83~5.17mmol/L. Abnormal cholesterol metabolism could easily lead to deposition of cholesterol in the blood vessel wall, forming atherosclerosis, causing coronary heart disease and stroke. |
|
Cholesterol content of human tissue |
Normal person of 70 kg weigh contains about 140 grams of cholesterol in the body; and its rough distribution is listed in the following table. The cholesterol content of various tissues is relatively stable. Moreover, the plasma concentration of cholesterol also keeps stable instead of being like fats and fatty acids which are prone to change. Table 1. The cholesterol content of various human tissues |
|
Classification |
Cholesterol in the body can be classified into free from and bound lipids form (cholesterol ester). |
|
Effect |
(1) It is involved in the formation of cell membranes. (2) It is the raw material for synthesis of bile acids, vitamin D and steroid hormones. (3) The total amount of serum cholesterol in China normal person serum is approximately 182.5 ± 4.3 mg%. Extra high blood cholesterol level indicates that cholesterol metabolism dysfunction may occur. Serum cholesterol level in patients with coronary atherosclerosis is often high. So the clinically determination of the serum cholesterol levels will help to diagnose certain diseases. |
|
Sources and absorption |
Source: (1) exogenous cholesterol coming from dietary. (2)From the body's own endogenous synthesis. Absorption: cholesterol is absorbed in the intestines and mainly synthesized in the liver, skin and the small intestine mucosa. Dietary cholesterol is mainly absorbed in the form of chylomicrons into the bloodstream. Cholesterol absorption also depends on the amount; the percentage of absorption is reduced upon a high intake of cholesterol. The absorption percentage is lower than 10% upon high intake amount. After free cholesterol is absorbed, 2/3 of them quickly binds to fatty acid and esterified to form cholesterol esters, making the ability of lipoproteins on carrying cholesterol be enhanced. After cholesterol enters into cells, it will be hydrolyzed and degreased by acidic lipase inside the lysosomes. A fraction of cholesterol in the cells is converted into steroid with excess cholesterol being directly discharged to the gut; another fraction of cholesterol is oxidized in the liver into bile acid and excreted together with the bile. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Transport |
There are two major lipoprotein involved in cholesterol transport: the low density lipoprotein (LDL) and high density lipoprotein (HDL). The former can transport cholesterol from the liver to whole body tissue cells with the later one transporting cholesterol from tissue cells back into the liver. |
|
Determination |
Serum cholesterol assays include measurement of total cholesterol (CT), free cholesterol (FC) and cholesterol ester (CE). |
|
Food containing high levels of cholesterol |
Animal foods contain high content of cholesterol, such as meat, eggs, milk and so on, but there is no cholesterol in plant foods, such as vegetables, fruits, legumes which almost does not contain any cholesterol, so high cholesterol people should avoid eating animal food with selecting plant food being a better choice. The cholesterol content of animal organs are particularly high, such as lung, kidney, liver, pig intestines, pig spleen, etc., they are high in cholesterol, so you should eat less. The cholesterol level in animal brain is also very high, especially in porcine brain, followed by bovine brain, sheep brain, brain duck, and chicken brain, etc. So to prevent high cholesterol, eat less of this kind of food. Food of low-cholesterol content: lean meat, rabbit meat, yellow croaker, hairtail, skinless chicken, carp, eel, ham square, white fish, jellyfish, milk, and sea cucumber. Food containing high levels of cholesterol (parentheses lists the number of milligrams of cholesterol per 100 grams of food contains). Animal brain has the highest cholesterol content: as porcine (3100 mg), bovine brain (2670 mg), sheep brain (2099 mg). Followed by yellow eggs: duck eggs as yellow (2110 mg), egg yolk (1705 mg), quail’s egg yolk (1674 mg), yellow egg (1132 mg). |
|
Harm of high cholesterol |
High cholesterol is clearly related to the occurrence of atherosclerosis. Modern molecular biology has showed that atherosclerotic lesions are initially begun with fatty streaks and atherosclerotic plaque disease, which is formed by macrophages which swallowed cholesterol and smooth muscle cells. On the other hand, high content of cholesterol, high blood pressure can cause harm to the integrity and function of vascular endothelium, resulting in a series of secondary damage. US National Cholesterol Education Program states: normal adult plasma cholesterol levels should be less than 5.2mmol/L; 5.2~6.2mmol/L is the high limit. For guys which exceed the upper limit should change their diet with further examination of high-density lipoprotein and low-density lipoprotein, and select related drugs for treatment. Clinical data have shown 8.5% drop in blood cholesterol and 12.6% decrease in LDL can reduce the mortality of coronary heart disease by 24% as well as reduce the incidence of myocardial infarction by 19%. |
|
Prevention |
(1) Low-fat diet. (2) Exercise. Saturated fatty acids cause the increase of serum cholesterol while unsaturated fatty acids reduce it. Mental workers have higher serum cholesterol levels than manual workers with exercise being able to reduce it. |
|
Production methods |
1. Extracted from pig brain (or spinal cord) or sheep brain. 2. Use the brain tissue of livestock as the raw materials. Preparation of dry powder: take fresh animal brain and spinal cord (remove the fat and spinal cord membranes), mince, dry at 40-50 °C to obtain the brain dry powder. Brain (pig, cattle, sheep) [40-50 °C] → brain dry powder Preparation of crude cholesterol crystals: brain powder is impregnated in 1.2 times the amount of acetone with constant stirring for extraction of 4.5h, and extract for continuously 6 times, filtrate, combine the extract, distill to recycle the acetone and obtain a yellow solid substance. Add 10 times the amount of ethanol, heat and reflux for 1h to obtain the cholesterol ethanol solution, filter and the filtrate is further cooled at 0-5 °C, stand static, separate out the crystals, filter gain to obtain the crude crystals of cholesterol. Brain dry powder [acetone] → yellow solid [ethanol] → cholesterol ethanol solution [0-5 °C] → crude cholesterol crystals. Making refined cholesterol: Take the crude cholesterol crystals and add 5 fold the amount of ethanol, add 5%-6% sulfuric acid, heat and reflux for 8h to obtained the hydrolysis liquid, further cool it at 0-5 °C, separate out the crystals, filter to obtain the crystals, add ethanol and wash to being neutral. The crystals washed into neutral were further added into 10 times the amount of 95% ethanol together with 3% activated carbon, heated for reflux and decolorization for 1h, insulate and filter, the filtrate was subject to cooling crystallization at 0-5 °C, repeated three times, filter, collect the crystals, compress for drying, evaporate the ethanol, and vacuum dry at 70-80 °C and obtain the refined cholesterol. The crude cholesterol crystals [ethanol, H2SO4] → [8h] hydrolysis liquid [0-5 °C] → crystal [ethanol, activated carbon] → [1h] refined cholesterol. 3. Bovine spinal cord is used as raw materials, extract it using petroleum ether and then repeatedly refine to obtain the product. |
|
Precautions |
Daily intake of cholesterol for normal adult should be less than 500 mg which is proper with patients suffering coronary heart disease should not exceed 300 mg. |
|
History |
Cholesterol was discovered in 1769 by Poulletier dela Salle (1719–1787), who isolated the compound from bile and gallstones. It was rediscovered by Michel Eugène Chevreul (1786–1889) in 1815 and named cholesterine. The name comes from the Greek words khole meaning bile and steros meaning solid or stiff . The “ine” ending was later changed to “ol” to designate it as an alcohol. |
|
Definition |
ChEBI: A cholestanoid consisting of cholestane having a double bond at the 5,6-position as well as a 3beta-hydroxy group. |
|
Production Methods |
The commercial material is normally obtained from the spinal cord of cattle by extraction with petroleum ethers, but it may also be obtained from wool fat. Purification is normally accomplished by repeated bromination. Cholesterol may also be produced by entirely synthetic means. Cholesterol produced from animal organs will always contain cholestanol and other saturated sterols. |
|
General Description |
Cholesterol is a minor sterol present in plants. It is majorly associated with the plant membranes and is a constituent of leaf surface lipids. |
|
Hazard |
Questionable carcinogen. |
|
Pharmaceutical Applications |
Cholesterol is used in cosmetics and topical pharmaceutical formulations at concentrations of 0.3–5.0% w/w as an emulsifying agent. It imparts water-absorbing power to an ointment and has emollient activity. Cholesterol also has a physiological role. It is the major sterol of the higher animals, and it is found in all body tissues, especially in the brain and spinal cord. It is also the main constituent of gallstones. |
|
Biochem/physiol Actions |
Cholesterol is a lipid that makes up about 20-25% of the structural components of the cell membranes. It determines the fluidity and permeability of the membrane, making it permeable to water but not to ions and protons. Cholesterol also regulates the functions of the transporters and signaling proteins present on the plasma membrane. The major sites of cholesterol synthesis are small intestine and liver. |
|
Safety Profile |
Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. Used in pharmaceutical and dermal preparations as an emulsifying agent. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Safety |
Cholesterol is generally regarded as an essentially nontoxic and nonirritant material at the levels employed as an excipient. It has, however, exhibited experimental teratogenic and reproductive effects, and mutation data have been reported. Cholesterol is often derived from animal sources and this must be done in accordance with the regulations for human consumption. The risk of bovine spongiform encephalopathy (BSE) contamination has caused some concern over the use of animalderived cholesterol in pharmaceutical products. However, synthetic methods of cholesterol manufacture have been developed. |
|
storage |
Cholesterol is stable and should be stored in a well-closed container, protected from light. |
|
Purification Methods |
Crystallise cholesterol from ethyl acetate, EtOH or isopropyl ether/MeOH. [Hiromitsu & Kevan J Am Chem Soc 109 4501 1987.] For extensive details of purification through the dibromide, see Fieser [J Am Chem Soc 75 5421 1953] and Schwenk and Werthessen [Arch Biochem Biophys 40 334 1952], and by repeated crystallisation from acetic acid; see Fieser [J Am Chem Soc 75 4395 1953]. Like many sterols, cholesterol gives colour reactions with conc H2SO4: When cholesterol is dissolved in a small volume of CHCl3 and mixed with conc H2SO4, the colour of the organic layer becomes crimson, then changes to purple and on further standing in air it turns to blue, then green and finally yellow. The H2SO4 layer develops a green fluorescence. [Beilstein 6 III 2607, 6 IV 4000.] |
|
Incompatibilities |
Cholesterol is precipitated by digitonin. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (injections; ophthalmic, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1
57-88-5 Relevant articles
Stereochemical Fate of the Isopropylidene Methyl Groups of Lanosterol during the Biosynthesis of Isofucosterol in Pinus Pinea
Nicotra, Francesco,Ronchetti, Fiamma,Russo, Giovanni,Lugaro, Giuseppe,Casellato, Marilena
, p. 498 - 502 (1981)
Lanosterol (16) was administered to Pinu...
Novel Trifunctional Building Blocks for Fluorescent Polymers
Batra, Dolly,Shea, Kenneth J.
, p. 3895 - 3898 (2003)
(Matrix presented) Herein, we describe t...
Hydrolysis of Steroidal Esters Catalysed by Tetracyanoethylene
Hanson, James R.,Uyanik, Cavit,Yildirim, Kudret
, p. 580 - 581 (1998)
Tetracyanoethylene has been shown to be ...
Me3SI-promoted chemoselective deacetylation: a general and mild protocol
Gurawa, Aakanksha,Kashyap, Sudhir,Kumar, Manoj
, p. 19310 - 19315 (2021/06/03)
A Me3SI-mediated simple and efficient pr...
Methods for preparing cholesterol, and derivatives and analogs thereof
-
Paragraph 0409-0410; 0419-0420, (2021/04/07)
The present invention relates to the fie...
Method for preparing cholesterol and derivatives thereof by taking phytosterol as raw material
-
Paragraph 0065, (2021/02/20)
The invention discloses a method for pre...
Method for synthesizing cholesterol by taking BA as raw material
-
Paragraph 0113-0117, (2021/08/14)
The invention discloses a method for syn...
57-88-5 Process route
-
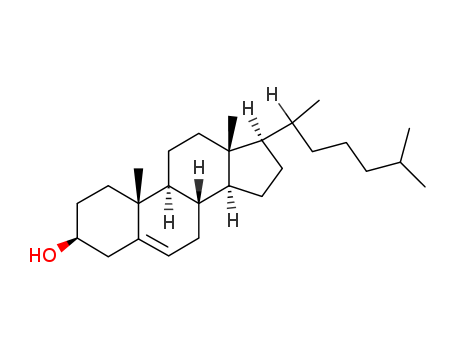
- 80239-44-7
1α-Hydroxy-5-cholesten-3-on

-
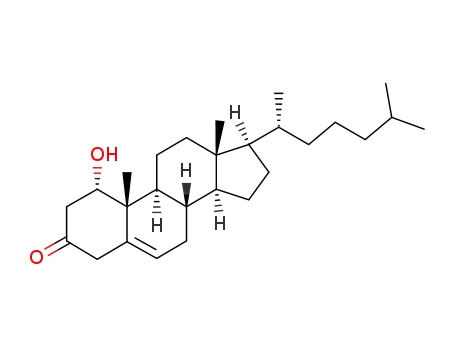
- 57-88-5
cholesterol

-
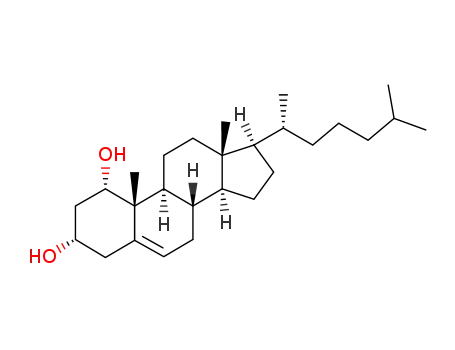
- 50392-20-6
5-Cholesten-1α,3α-diol

-
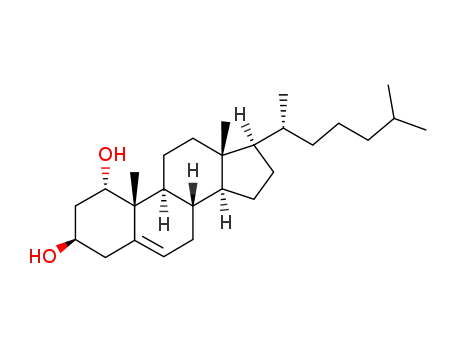
- 26358-75-8
1α,3β-dihydroxycholest-5-ene
| Conditions | Yield |
|---|---|
|
With lithium; ammonium chloride; In diethyl ether; ammonia; at -33 ℃; for 0.5h; Yield given. Yields of byproduct given;
|
-
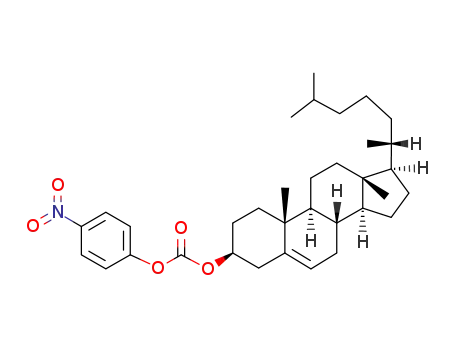
- 79824-96-7
(4-nitro-phenyl)-cholesteryl carbonate

-
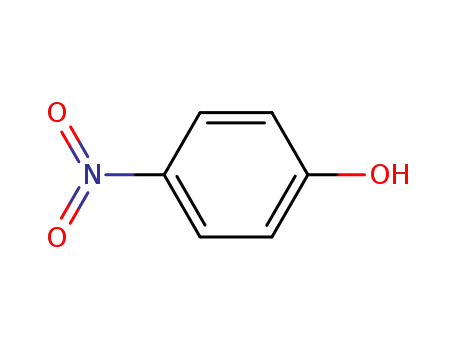
- 100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
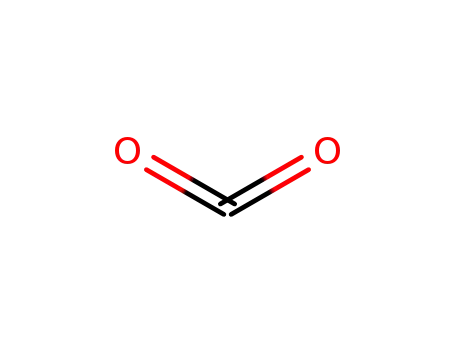
- 124-38-9,18923-20-1
carbon dioxide

-

- 57-88-5
cholesterol
| Conditions | Yield |
|---|---|
|
With cloned RNA; saline buffer; In tetrahydrofuran; at 37 ℃; for 72h; pH=7.5; Enzyme kinetics;
|
57-88-5 Upstream products
-
604-35-3
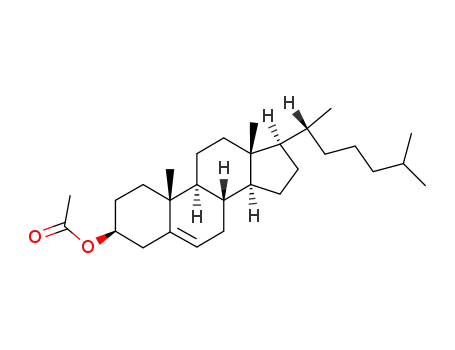
Cholesteryl acetate
-
25092-65-3
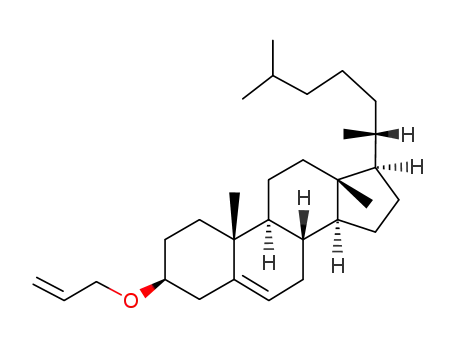
allyl cholesteryl ether
-
1857-80-3
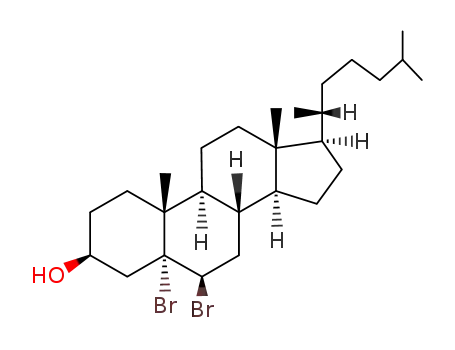
5α,6β-dibromocholestan-3β-ol
-
1182-65-6
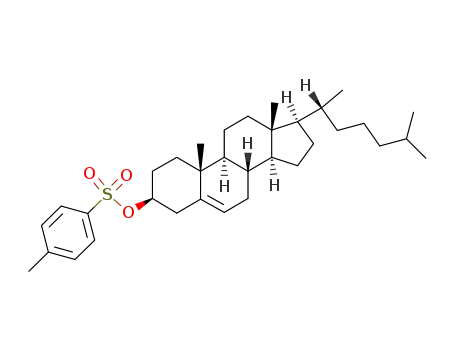
cholesteryl p-toluenesulfonate
57-88-5 Downstream products
-
6252-45-5
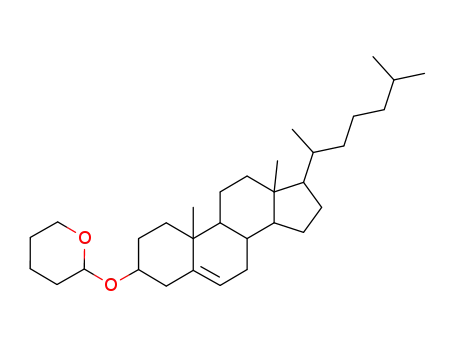
Cholesterol-3-tetrahydropyranylether
-
2930-80-5

17-(1,5-Dimethyl-hexyl)-3-iodo-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene
-
1856-05-9
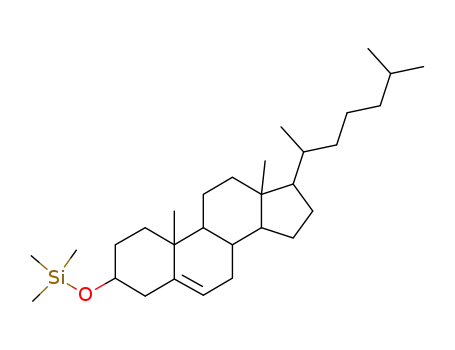
Trimethylsilylaether von Cholesterol
-
601-54-7

cholest-5-en-3-one
Relevant Products
-
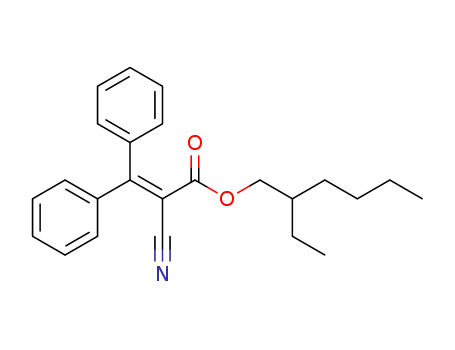
0ctocrylene
CAS:6197-30-4
-
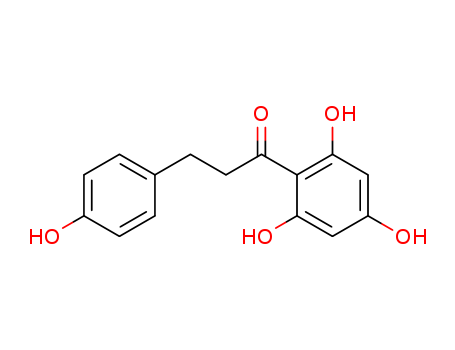
Phloretin
CAS:60-82-2
-
Sodium Acetylated Hyaluronate
CAS:158254-23-0