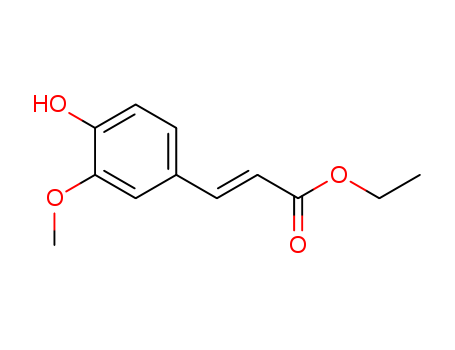
4046-02-0
- Product Name:Ethyl Ferulic Acid
- Molecular Formula:C12H14O4
- Purity:99%
- Molecular Weight:222.241
Product Details
pd_meltingpoint:63-65 °C(lit.)
Manufacturer Supply Best Quality Ethyl Ferulic Acid 4046-02-0 with Efficient Transportation
- Molecular Formula:C12H14O4
- Molecular Weight:222.241
- Vapor Pressure:2.17E-06mmHg at 25°C
- Melting Point:63-65 °C(lit.)
- Refractive Index:1.565
- Boiling Point:382.3 °C at 760 mmHg
- PKA:8.88±0.18(Predicted)
- Flash Point:132.5 °C
- PSA:55.76000
- Density:1.173 g/cm3
- LogP:1.97710
Ethyl 4'-hydroxy-3'-methoxycinnamate(Cas 4046-02-0) Usage
InChI:InChI=1/C12H14O4/c1-3-16-12(14)7-5-9-4-6-10(13)11(8-9)15-2/h4-8,13H,3H2,1-2H3/b7-5+
4046-02-0 Relevant articles
Identification of 8-O-4/8-5(Cyclic)- and 8-8(Cyclic)/5-5-Coupled Dehydrotriferulic Acids, Naturally Occurring in Cell Walls of Mono- and Dicotyledonous Plants
Waterstraat, Martin,Bunzel, Diana,Bunzel, Mirko
, p. 7244 - 7250 (2016)
Besides ferulate dimers, higher oligomer...
Production of feruloylated lysophospholipids via a one-step enzymatic interesterification
Rychlicka, Magdalena,Maciejewska, Gabriela,Niezgoda, Natalia,Gliszczyńska, Anna
, (2020)
Incorporation of ferulic acid (FA) into ...
Rapid syntheses of dehydrodiferulates via biomimetic radical coupling reactions of ethyl ferulate
Lu, Fachuang,Wei, Liping,Azarpira, Ali,Ralph, John
, p. 8272 - 8277 (2012)
Dehydrodimerization of ferulates in gras...
Peroxidase-catalyzed oligomerization of ferulic acid esters
Bunzel, Mirko,Heuermann, Birgit,Kim, Hoon,Ralph, John
, p. 10368 - 10375 (2008)
Valuable information about possible type...
First stereoselective and concise synthesis of rhoiptelol C
Purushotham Reddy, Sudina,Chinnababu, Baggu,Venkateswarlu, Yenamandra
, p. 999 - 1003 (2014)
The first concise stereoselective total ...
Lipase-catalyzed preparation of mono- and diesters of ferulic acid
Sandoval, Georgina,Quintana, Paula G.,Baldessari, Alicia,Ballesteros, Antonio O.,Plou, Francisco J.
, p. 89 - 97 (2015)
Lipophilic and stable derivatives of fer...
Synthesis of Novel Antiviral Ferulic Acid-Eugenol and Isoeugenol Hybrids Using Various Link Reactions
Gan, Xiuhai,Wang, Zhengxing,Hu, Deyu
, p. 13724 - 13733 (2021/11/23)
To develop novel antiviral agents, some ...
Ruthenium-catalyzed intramolecular arene C(sp2)-H amidation for synthesis of 3,4-dihydroquinolin-2(1 H)-ones
Au, Chi-Ming,Ling, Cho-Hon,Sun, Wenlong,Yu, Wing-Yiu
supporting information, p. 3310 - 3314 (2021/05/29)
We report the [Ru(p-cymene)(l-proline)Cl...
Solvent role in the lipase-catalysed esterification of cinnamic acid and derivatives. Optimisation of the biotransformation conditions
Suárez-Escobedo, Laura,Gotor-Fernández, Vicente
, (2021/02/05)
The esterification of cinnamic acid has ...
First total syntheses of four natural bioactive glucosides
Xu, Guangya,Wu, Min,Yao, Zhongquan,Lou, Hongbin,Du, Weihong,Song, Mingwei,He, Yujiao,Dong, Hongbo
supporting information, p. 1266 - 1271 (2021/02/06)
The efficient total syntheses of four bi...
4046-02-0 Process route
-
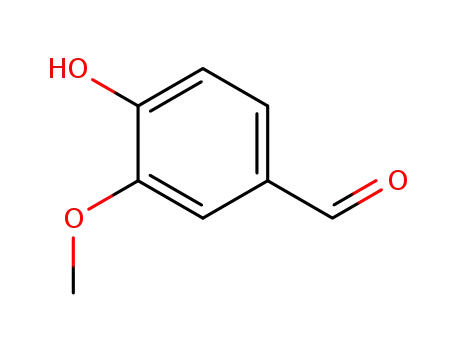
- 121-33-5,8014-42-4
vanillin

-
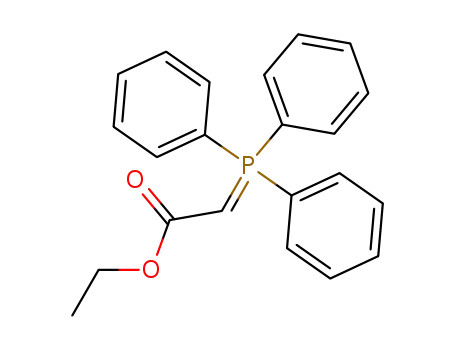
- 1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
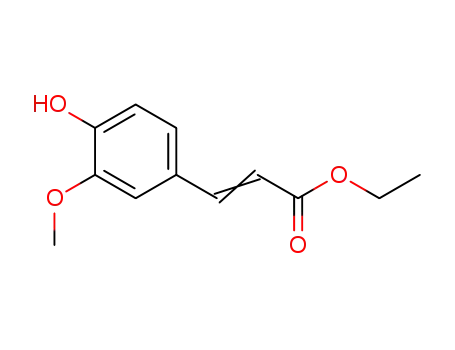
- 28028-62-8,74257-26-4,4046-02-0
ethyl ferulate
| Conditions | Yield |
|---|---|
|
In dichloromethane; at 20 ℃; for 4h;
|
97% |
|
In benzene; at 80 ℃; for 4h;
|
95% |
|
In chloroform; for 5h; Reflux;
|
91% |
|
In dichloromethane; at 20 ℃;
|
85.9% |
|
In toluene; for 2h; Heating / reflux;
|
80% |
|
In toluene; at 80 ℃; for 4h;
|
|
|
Reflux;
|
|
|
In toluene; at 110 ℃; for 3h;
|
5.73 g |
|
In dichloromethane; at 0 - 20 ℃;
|
-
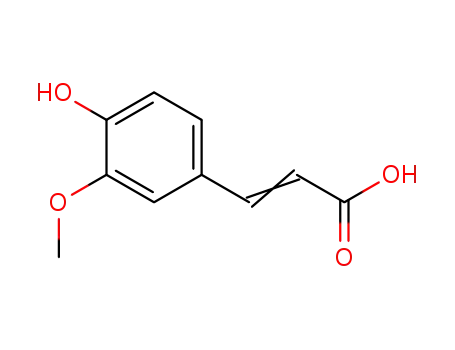
- 1135-24-6,537-98-4
3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
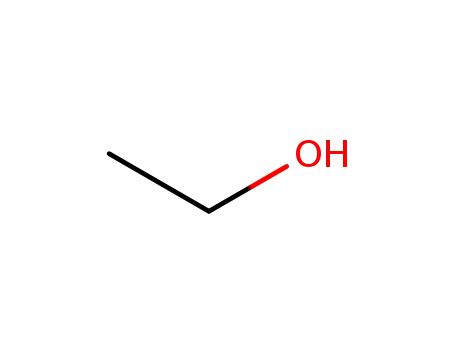
- 64-17-5
ethanol

-

- 28028-62-8,74257-26-4,4046-02-0
ethyl ferulate
| Conditions | Yield |
|---|---|
|
at 50 ℃; for 76h;
|
100% |
|
With hydrogenchloride; In water; for 48h; Reflux;
|
99% |
|
With thionyl chloride; Heating;
|
90% |
|
Microwave irradiation;
|
81% |
|
Reflux; Acidic conditions;
|
78% |
|
With sulfuric acid; sodium sulfate; at 65 - 85 ℃; for 6h;
|
77.4% |
|
With sulfuric acid;
|
67.5% |
|
With Novozym 435 from Candida antarctica; In tert-butyl alcohol; at 60 ℃; for 312h; Further Variations:; Temperatures; Reaction partners; Reagents; reaction time; Product distribution;
|
20 % Turnov. |
|
With Novozym 435 from Candida antarctica; In tert-butyl alcohol; at 60 ℃; for 312h;
|
20 % Turnov. |
|
In hydrogenchloride; water; at 37 ℃; for 24h;
|
|
|
With thionyl chloride;
|
|
|
With thionyl chloride; at 20 ℃; for 2h; Reagent/catalyst; Temperature; Time;
|
|
|
With sulfuric acid; for 2h; Reflux;
|
68.2 %Chromat. |
|
With sulfuric acid; for 12h; Reflux;
|
|
|
Acidic conditions;
|
|
|
With sulfuric acid; Reflux;
|
4046-02-0 Upstream products
-
867-13-0
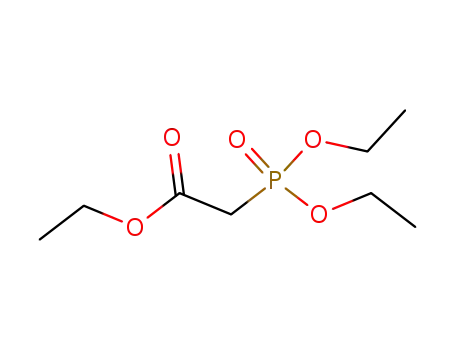
diethoxyphosphoryl-acetic acid ethyl ester
-
121-33-5

vanillin
-
1135-24-6

3-(4-hydroxy-3-methoxyphenyl)acrylic acid
-
64-17-5

ethanol
4046-02-0 Downstream products
-
61292-90-8
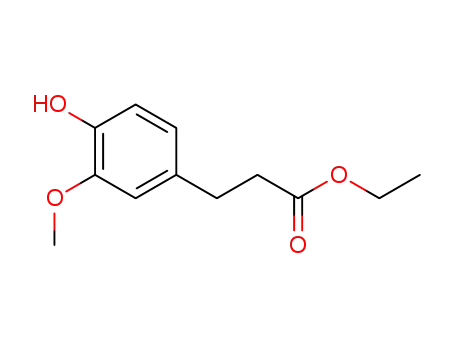
ethyl 3-(4-hydroxy-3-methoxyphenyl)propanoate
-
458-35-5
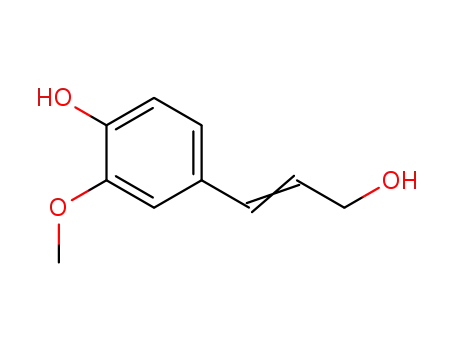
coniferol
-
515835-74-2
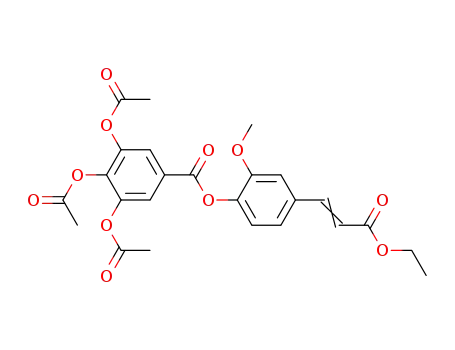
ethyl 3-[4-(3,4,5-triacetyloxybenzoyloxy)-3-methoxyphenyl]-2-propenoate
-
94930-75-3
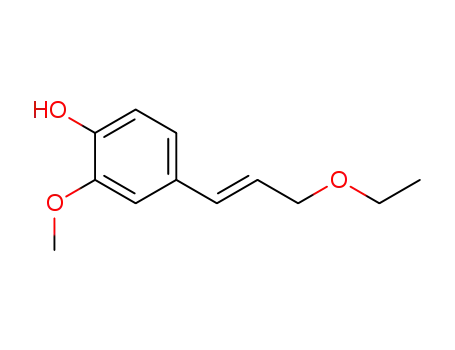
(E)-coniferyl ethyl ether
Relevant Products
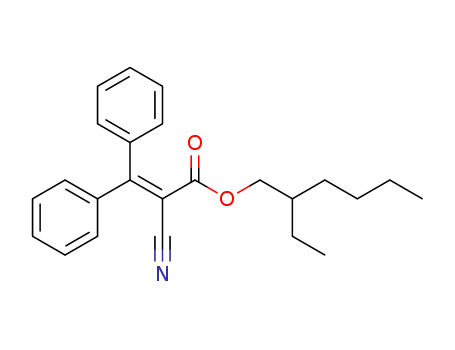
-
0ctocrylene
CAS:6197-30-4
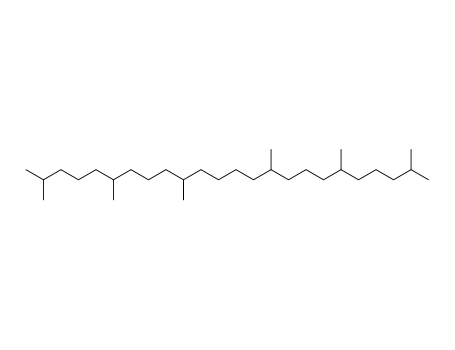
-
Squalane
CAS:111-01-3
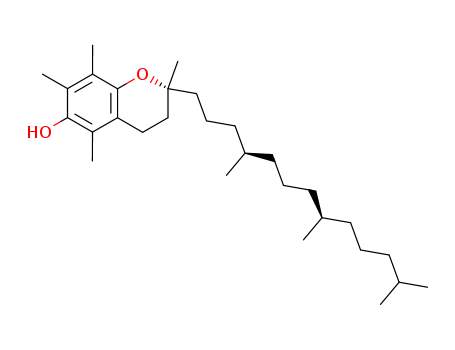
-
Mixed Tocopherol Oil
CAS:10191-41-0