79-81-2
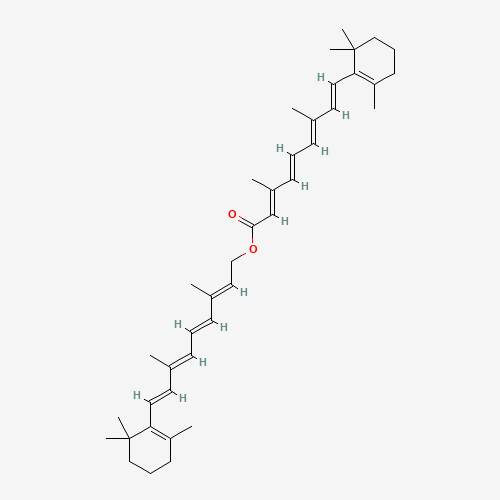
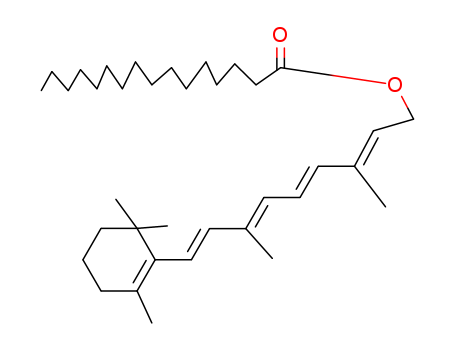
- Product Name:Vitamin A palmitate
- Molecular Formula:C36H60O2
- Purity:99%
- Molecular Weight:524.871
Product Details
pd_meltingpoint:2-8 °C
Buy Quality Vitamin A palmitate 79-81-2 In Stock with Immediately Delivery
- Molecular Formula:C36H60O2
- Molecular Weight:524.871
- Vapor Pressure:0mmHg at 25°C
- Melting Point:2-8 °C
- Refractive Index:1.511
- Boiling Point:607.5 °C at 760 mmHg
- Flash Point:79.7 °C
- PSA:26.30000
- Density:0.92 g/cm3
- LogP:11.54250
Retinol palmitate(Cas 79-81-2) Usage
|
General Description |
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.Retinyl palmitate belongs to a category of compounds called retinoids, which are chemically similar to vitamin A. It exhibits a beneficial effect on vision, skin and immune function, inhibits cell proliferation and prevents cancer. It is an important dietary as well as a therapeutic compound. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
Review: Vitamin A metabolism. |
|
Safety Profile |
Mildly toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
The palmitate is separated from retinol by column chromatography on water-deactivated alumina with hexane containing a very small percentage of acetone. It is also chromatographed on TLC silica gel G, using pet ether/isopropyl ether/acetic acid/water (180:20:2:5) or pet ether/acetonitrile/acetic acid/water (190:10:1:15) to develop the chromatogram. It is then recrystallised from propylene at low temperature (below -47o). [Beilstein 6 IV 4135.] |
InChI:InChI=1/C36H58O3/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-25-34(37)39-35(38)29-31(3)23-20-22-30(2)26-27-33-32(4)24-21-28-36(33,5)6/h20,22-23,26-27,29H,7-19,21,24-25,28H2,1-6H3/b23-20+,27-26+,30-22+,31-29+
79-81-2 Relevant articles
Antioxidant activity and synergism of butylhydroxytoluene in processes of oxidation of retinol esters
Finkel'shtein,Mednikova,Alekseev,Kozlov
, p. 100 - 104 (1977)
-
Synthesis of retinyl palmitate catalyzed by Candida sp.99-125 lipase immobilized on fiber-like SBA-15 mesoporous material
Zhu, Kai,Wang, Jianqiang,Wang, Yan-Hua,Liu, Hui,Han, Ping-Fang,Wei, Ping
, p. 7593 - 7602 (2011)
Candida sp.99-125 lipase was suitable fo...
Synthesis method of 4-palmitoyloxy-2-methyl-2-butenal and synthesis method of vitamin A palmitate
-
Paragraph 0025; 0073-0077, (2021/04/10)
The invention discloses a synthesis meth...
Method for preparing vitamin A and vitamin A ester
-
Paragraph 0067; 0072, (2020/04/17)
The invention provides a novel method fo...
Preparation method of vitamin A ester intermediate C15 and vitamin A ester
-
, (2020/08/18)
The invention provides a preparation met...
Preparation method of vitamin A palmitate
-
Paragraph 0017-0028, (2018/10/27)
The invention belongs to the technical f...
79-81-2 Process route
-
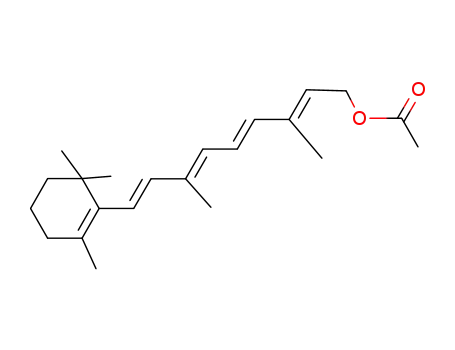
- 127-47-9,64536-04-5
Retinol acetate

-
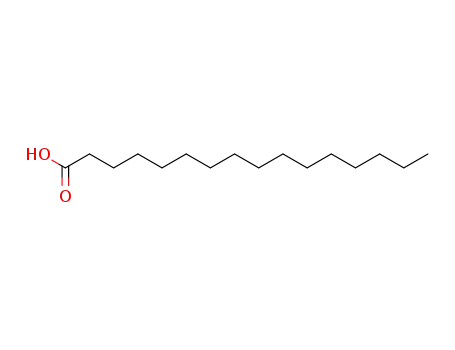
- 57-10-3
1-hexadecylcarboxylic acid

-
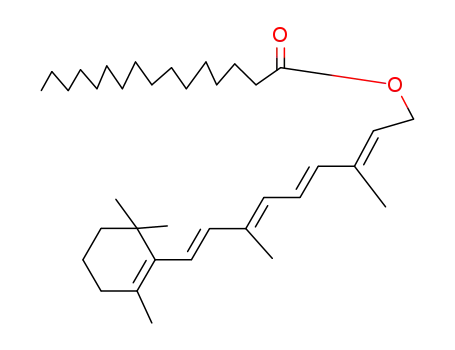
- 79-81-2
retinyl palmitate

-
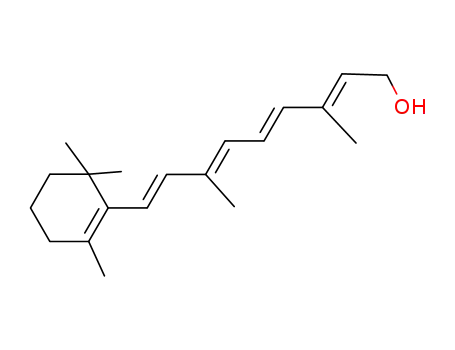
- 68-26-8
RETINOL
| Conditions | Yield |
|---|---|
|
With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21; In toluene; at 20 ℃; for 15h; Product distribution / selectivity; Enzymatic reaction;
|
78% |
-
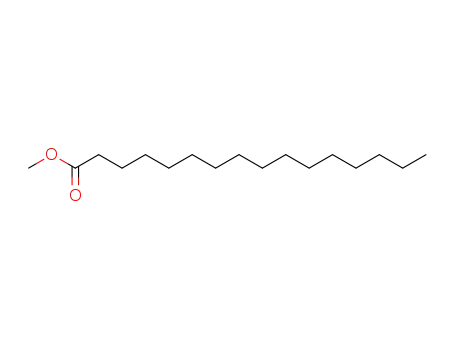
- 112-39-0
hexadecanoic acid methyl ester

-

- 127-47-9,64536-04-5
Retinol acetate

-

- 79-81-2
retinyl palmitate
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In methanol; at 55 ℃; for 3h; under 11.2511 - 16.5017 Torr; Concentration; Pressure; Large scale;
|
96% |
79-81-2 Upstream products
-
110-86-1
pyridine
-
68-26-8
RETINOL
-
112-67-4
n-hexadecanoyl chloride
-
108-88-3
toluene
79-81-2 Downstream products
-
68-26-8
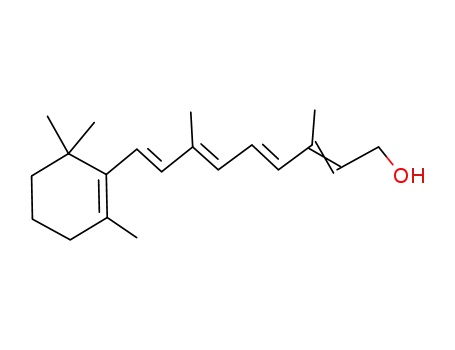
retinol
-
4759-48-2
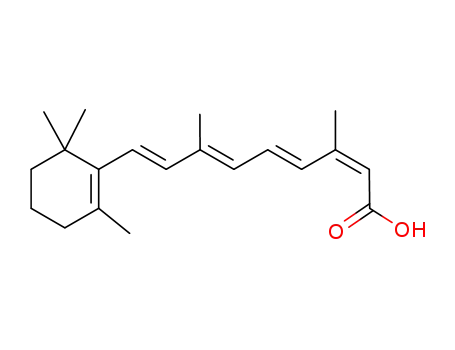
13-cis-retinoic acid
-
302-79-4
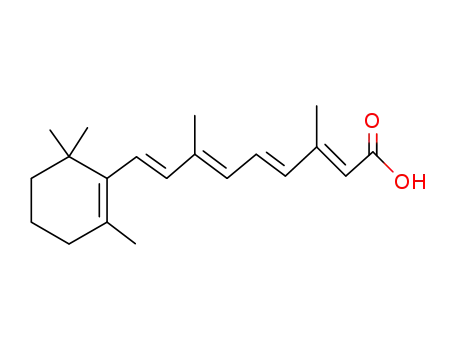
all-trans-retinoic-acid
-
3899-20-5
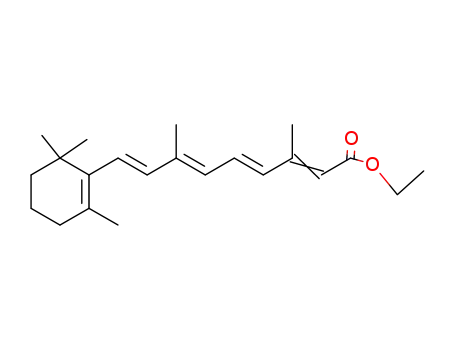
ethyl retinoate
Relevant Products
-
0ctocrylene
CAS:6197-30-4
-
Ferulic Acid
CAS:1135-24-6
-
Retinyl Retinoate
CAS:15498-86-9