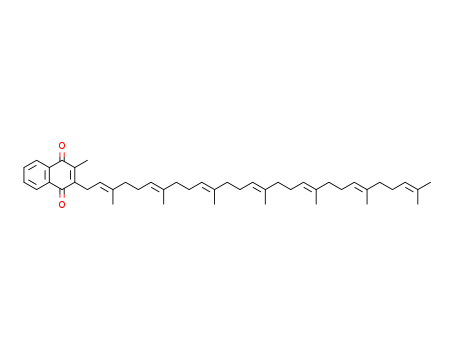
2124-57-4
- Product Name:Vitamin K2-MK7
- Molecular Formula:C46H64O2
- Purity:99%
- Molecular Weight:649.013
Product Details
pd_meltingpoint:54 °C
Quality Factory Supply 99% Pure Vitamin K2-MK7 2124-57-4 with Efficient Delivery
- Molecular Formula:C46H64O2
- Molecular Weight:649.013
- Vapor Pressure:1.38E-20mmHg at 25°C
- Melting Point:54 °C
- Refractive Index:1.532
- Boiling Point:720.1 °C at 760mmHg
- Flash Point:254.9 °C
- PSA:34.14000
- Density:0.961 g/cm3
- LogP:14.09740
Vitamin K2(35)(Cas 2124-57-4) Usage
|
Definition |
ChEBI: A menaquinone whose side-chain contains seven isoprene units in an all-trans-configutation. |
|
General Description |
Vitamin K2, also known as menaquinone, is a polyisoprenoid-substituted?napthoquinone. Menaquinone-7 (MK-7) is a subtype of vitamin K2. It is found in animals, soy protein, fermented products and is primarily produced by bacteria.Vitamin K2 (MK-7)-(5,6,7,8-d4,2-methyl-d3) is a deuterated vitamin K2 wherein C-5, C-6, C-7, C-8, and 2-methyl protons are replaced by deuterium. |
InChI:InChI=1/C46H64O2/c1-34(2)18-12-19-35(3)20-13-21-36(4)22-14-23-37(5)24-15-25-38(6)26-16-27-39(7)28-17-29-40(8)32-33-42-41(9)45(47)43-30-10-11-31-44(43)46(42)48/h10-11,18,20,22,24,26,28,30-32H,12-17,19,21,23,25,27,29,33H2,1-9H3/b35-20+,36-22+,37-24+,38-26+,39-28+,40-32+
2124-57-4 Relevant articles
PROCESS OF VITAMIN K2 DERIVATIVES PREPARATION
-
Page/Page column 22-24, (2021/04/17)
The invention relates to an improved pro...
Menaquinol Compositions and Methods of Treatment
-
Paragraph 0129-0130, (2020/04/09)
The present application discloses method...
PROCESS OF VITAMIN K2 DERIVATIVES PREPARATION
-
, (2019/10/29)
Provided is an improved process of vitam...
Convergent Synthesis of Menaquinone-7 (MK-7)
Baj, Aneta,Wa?ejko, Piotr,Kutner, Andrzej,Kaczmarek, ?ukasz,Morzycki, Jacek W.,Witkowski, Stanis?aw
, p. 1026 - 1033 (2016/11/11)
A practical synthesis of menaquinone-7 (...
2124-57-4 Process route
-
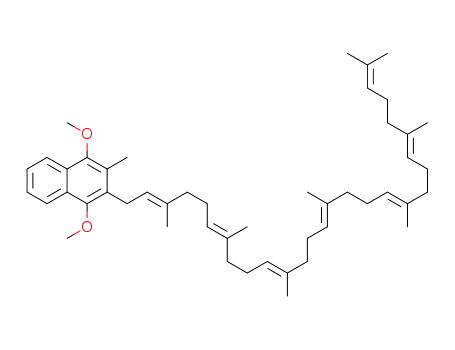
- 1218784-62-3
2-(3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaenyl)-1,4-dimethoxy-3-methylnaphthalene

-
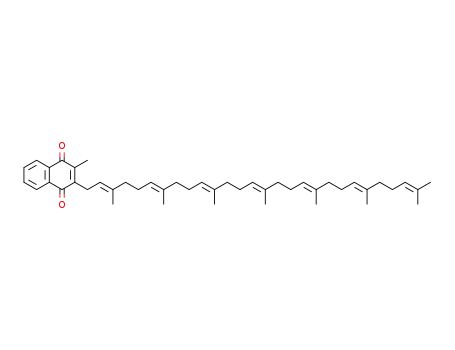
- 2124-57-4,27670-94-6
menaquinone-7
| Conditions | Yield |
|---|---|
|
With ammonium cerium (IV) nitrate; In water; acetonitrile; at 20 ℃; for 4.08333h;
|
80% |
|
With ammonium cerium (IV) nitrate; In acetonitrile; at 20 ℃; for 4.08333h;
|
80% |
|
With ammonium cerium (IV) nitrate; In water; acetonitrile; at 0 ℃; for 0.75h;
|
72% |
|
With ammonium cerium (IV) nitrate; In dichloromethane; water; acetonitrile; at 0 ℃; for 0.75h;
|
0.753 g |
-
- 1597486-88-8
C50H74O2

-

- 2124-57-4,27670-94-6
menaquinone-7
| Conditions | Yield |
|---|---|
|
With ammonium cerium (IV) nitrate; In dichloromethane; water; acetonitrile; at 0 ℃; for 0.25h;
|
72% |
2124-57-4 Upstream products
-
4128-17-0
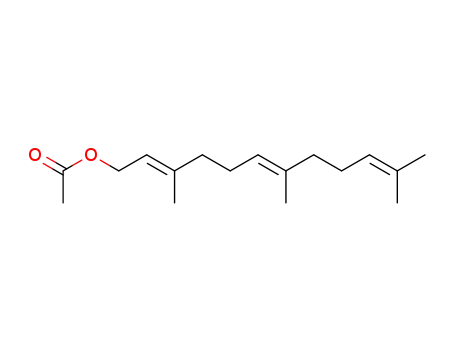
(2E,6E)-farnesyl acetate
-
93787-91-8
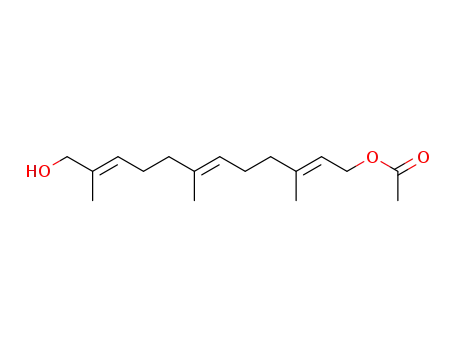
E,E,E-12-hydroxyfarnesyl acetate
-
1597486-72-0
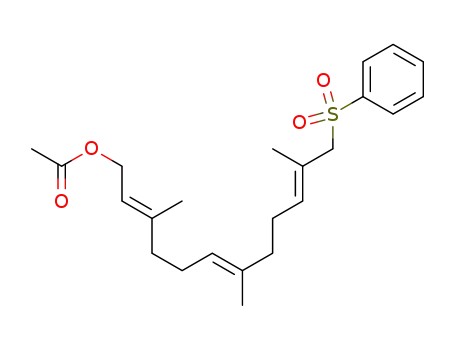
(2E,6E,10E)-3,7,11-trimethyl-12-(phenylsulfonyl)-dodeca-2,6,10-trien-1-yl acetate
-
24163-93-7

farnesyl bromide
2124-57-4 Downstream products
-
1453189-02-0
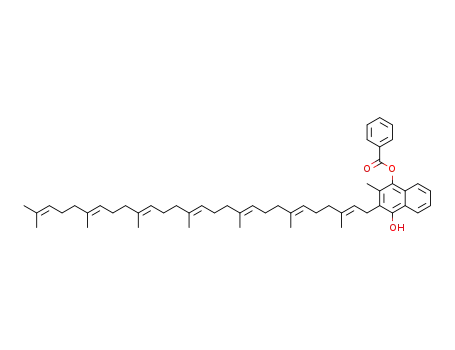
3-((2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaen-1-yl)-4-hydroxy-2-methylnaphthalen-1-yl benzoate
-
1453189-03-1
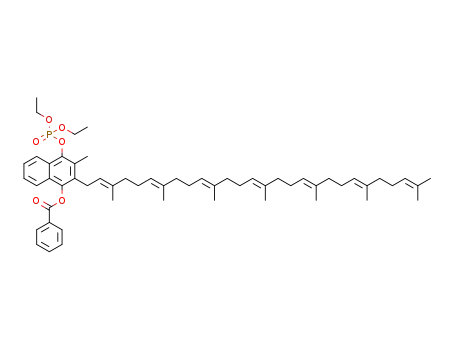
4-((diethoxyphosphoryl)oxy)-2-((2E,6E, 10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaen-1-yl)-3-methylnaphthalen-1-yl benzoate
-
1453189-04-2
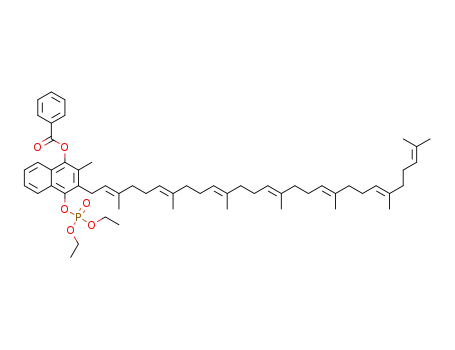
4-((diethoxyphosphoryl)oxy)-3-((2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaen-1-yl)-2-methylnaphthalen-1-yl benzoate
-
1453189-05-3
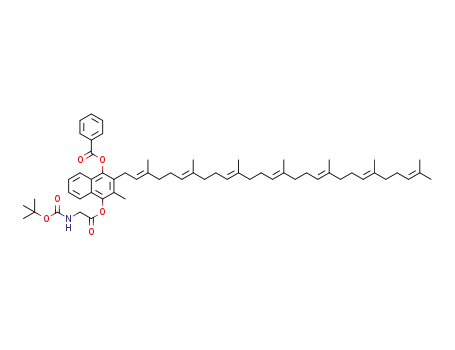
4-(((tert-butoxycarbonyl)glycyl)oxy)-2-((2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaen-1-yl)-3-methylnaphthalen-1-yl benzoate
Relevant Products
-
0ctocrylene
CAS:6197-30-4
-
Hydroxypinacolone Retinoate
CAS:893412-73-2
-
Tocopheryl Glucoside
CAS:104832-72-6