116-31-4
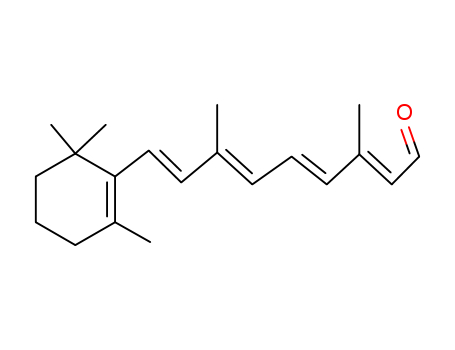
- Product Name:Retinaldehyde
- Molecular Formula:C20H28O
- Purity:99%
- Molecular Weight:284.442
Product Details
pd_meltingpoint:61-63 °C
Appearance:Yellow powder
Factory Supply Industrial Grade Retinaldehyde 116-31-4 with Best Price
- Molecular Formula:C20H28O
- Molecular Weight:284.442
- Appearance/Colour:Yellow powder
- Vapor Pressure:2.61E-07mmHg at 25°C
- Melting Point:61-63 °C
- Refractive Index:1.54
- Boiling Point:421.4°C at 760 mmHg
- Flash Point:205.4°C
- PSA:17.07000
- Density:0.948g/cm3
- LogP:5.71690
ALL-TRANS-RETINAL(Cas 116-31-4) Usage
|
Synthesis Reference(s) |
Tetrahedron Letters, 29, p. 419, 1988 DOI: 10.1016/S0040-4039(00)80111-2 |
|
Biological Activity |
all-trans retinal, also known as vitamin a aldehyde or retinaldehyde, is one of the many forms of vitamin a and also the oxidation product of all-trans retinol [1]. all-trans retinal are associated with one of the two isoforms of cellular retinol-binding proteins (crbp-i and crbp-ii) with kd values of 50 and 90 nm, respectively [1].crbp-i and crbp-ii were the first intracellular retinoid-binding proteins. both proteins display a similar binding affinity towards retinal. they play important roles in retinoid biology and regulation of the metabolism of retinol and retinal. crbp-i is used to regulate vitamin a storage and synthesis of retinoic acid. and crbp-ii has a role in the initial processing of retinol from food [1].all-trans retinal is one form of vitamin a. all-trans retinal, the initial substrate of retinoid cycle, is a chemically reactive aldehyde that can form toxic conjugates with proteins and lipids, leading to degeneration of the retina [2]. |
|
Biochem/physiol Actions |
All-trans retinal is converted to retinoic acid in vivo by the action of retinal dehydrogenase. Retinoic acid is a ligand for both the retinoic acid receptor (RAR) and the retinoid X receptor (RXR) that act as transcription factors to regulate the growth and differentiation of normal and malignant cells. Retinal isomers are also chromophores that bind to opsins, a family of G-protein-linked transmembrane proteins, to form photosensitive receptors in visual and nonvisual systems. All-trans retinal is a potent photosensitizer. |
|
Purification Methods |
The aldehyde is separated from retinol by column chromatography on water-deactivated alumina. Elute with 1-2% acetone in hexane, or on TLC plates of silica gel G and using the same eluting solvent. It crystallises from pet ether or n-hexane as yellow-orange crystals, and the UV in hexane has max at 373nm (A1cm 1% 1,548) and 368nm ( 48,000). It is an irritant and is light sensitive. Store it in sealed ampoules under N2. The semicarbazone forms yellow crystals from CHCl3/Et2O or EtOH, m 199-201o(dec). The 9-cis-isomer [514-85-2] and the 13-cis-isomer [472-86-6] [max at 375nm ( 1,250) in EtOH] are also available commercially. [Beilstein 7 III 1742.] |
|
references |
[1]. noy n. retinoid-binding proteins: mediators of retinoid action. biochem j. 2000 jun 15;348 pt 3:481-95.[2]. kiser pd, golczak m, maeda a, et al. key enzymes of the retinoid (visual) cycle in vertebrate retina. biochim biophys acta. 2012 jan;1821(1):137-51. |
|
Definition |
ChEBI: A retinal in which all four exocyclic double bonds have E- (trans-) geometry. |
|
General Description |
All trans-Retinal is one of the major derivatives of vitamin A group. A variety of food serves as a source of vitamin A. It is predominant in liver and among the brightly colored vegetables. |
InChI:InChI=1/C20H28O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,15H,7,10,14H2,1-5H3/b9-6+,12-11+,16-8-,17-13+
116-31-4 Relevant articles
The OSM (Oxidation State Modification) Concept: Application to a New and Rapid Synthesis of Retinoids
Duhamel, Lucette,Duhamel, Pierre,Ancel, Jean-Erick
, p. 1209 - 1210 (1994)
The OSM (oxidation state modification) c...
Triplet quantum chain process in the photoisomerization of 9-cis retinal as revealed by nanosecond time-resolved infrared spectroscopy
Yuzawa, Tetsuro,Hamaguchi, Hiro-o
, p. 414 - 418 (2010)
The mechanism of the photoisomerization ...
Mechanism for the Two-bond Isomerization in the Photoirradiation of 7,9-Di-cis-retinal
Liu, Robert S. H.,Zhu, Yun
, p. 1765 - 1766 (1993)
The two-bond isomerization process of 7,...
-
Karrer,Rueegger
, p. 284 (1940)
-
Efficient, low-cost synthesis of retinal (Vitamin A aldehyde)
Hruszkewycz, Damian P.,Cavanaugh, Kathryn R.,Takamura, Kathryn T.,Wayman, Lora M.,Curley Jr., Robert W.
, p. 2205 - 2207 (2011)
Inexpensive retinyl acetate has been sub...
Retinal isomer composition in some bacteriorhodopsin mutants under light and dark adaptation conditions
Song,Yang,El-Sayed,Lanyi
, p. 10052 - 10055 (1995)
The isomeric composition of retinal was ...
Photophysical and Photochemical Behavior of 11-cis-Retinal and Its Schiff Base in a Micelle
Becker, Ralph S.,Freedman, Kenn,Lenoble, Christian
, p. 4334 - 4336 (1986)
The photophysical and photochemical beha...
Exploratory study of β-carotene autoxidation
Mordi,Walton,Burton,Hughes,Ingold,Lindsay
, p. 4203 - 4206 (1991)
The main products in the early stages of...
Photoisomerization of polyenes. 30. Quantum chain processes in photoisomerization of the all-trans, 7-cis, and 11-cis isomers of retinal
Ganapathy,Liu
, p. 3459 - 3464 (1992)
-
Prenylation reaction performed with catalytically generated potassium prenal dienolate
Cahard, Dominique,Duhamel, Lucette,Lecomte, Sandrine,Poirier, Jean-Marie
, p. 1399 - 1401 (1998)
A new prenylation method based on the re...
146. Retro-Aldol Reaction of Retinylidene-1,3-Diketones; Correlation with Biological Activity
Acton, Nancy,Brossi, Arnold
, p. 1396 - 1399 (1980)
Retro-Aldol reaction of retinylidene-dim...
Polyunsaturated aldehydes by direct polyvinylogation of carbonyl compounds using functionalized phosphonates.
Duhamel, Lucette,Guillemont, Jerome,Gallic, Yann Le,Ple, Gerard,Poirier, Jean-Marie,et al.
, p. 3129 - 3132 (1990)
Carbonyl compounds are converted into po...
Silica Gel Mediated Photoisomerization of Retinal Isomers and Comparisons with Other Forms of Environmental Pertubation
Zawadzki, Mary E.,Ellis, Arthur B.
, p. 3156 - 3161 (1983)
The electronic spectra and photoreactivi...
Reactivity of retinoids and carotenoids in autoxidation
Finkelshtein,Krasnokutskaya
, p. 411 - 418 (1996)
The autoxidation of various retinyl poly...
Palladium-catalysed vinylation of tertiary allylic alcohols: A new protocol for the synthesis of isoprenoid aldehydes.
Bienayme,Yezeguelian
, p. 3389 - 3396 (1994)
Heck vinylation of tertiary allylic alco...
Preparation and characterization of metal-substituted carotenoid cleavage oxygenases
Sui, Xuewu,Farquhar, Erik R.,Hill, Hannah E.,von Lintig, Johannes,Shi, Wuxian,Kiser, Philip D.
, p. 887 - 901 (2018)
Carotenoid cleavage oxygenases (CCO) are...
Studies on the Catalyzed Interconversions of Vitamin A Derivatives
Rando, Robert R.,Chang, Andrew
, p. 2879 - 2882 (1983)
The kinetics of the I2-catalyzed isomeri...
Cyclopropyl and isopropyl derivatives of 11- cis and 9- cis retinals at C-9 and C-13: Subtle steric differences with major effects on ligand efficacy in rhodopsin
DeGrip, Willem J.,Bovee-Geurts, Petra H. M.,Wang, Yajie,Verhoeven, Michiel A.,Lugtenburg, Johan
, p. 383 - 390 (2011)
Retinal is the natural ligand (chromopho...
Configurational Changes of Retinal in the Triplet State: Picosecond Time-Resolved Absorption Spectroscopy on the 7-Cis, 11-Cis, and 13-Cis Isomers and High-Performance Liquid Chromatography Analysis of Photoisomerization
Mukai, Yumiko,Koyama, Yasushi,Hirata, Yoshinori,Mataga, Noboru
, p. 4649 - 4653 (1988)
The triplet state of retinal was produce...
An improved synthesis of retinal (Vitamin A Aldehyde)
Sacolick, Davidson A.,Curley, Robert W.
, p. 325 - 327 (2013)
-
Broad-spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative
Kesel, Andreas J.
, p. 1656 - 1664 (2011)
Aromatic aldehyde-derived thiosemicarbaz...
Urea unfolding of opsin in phospholipid bicelles
McKibbin, Craig,Farmer, Nicola A.,Edwards, Patricia C.,Villa, Claudio,Booth, Paula J.
, p. 494 - 500 (2009)
Opsin is the unstable apo-protein of the...
Monolayer films of retinal-1 and the effects of light on them.
Maeda,Isemura
, p. 765 - 766 (1967)
-
Reaction of retinol with peroxynitrite
Suzuki, Rie,Kulkarni, Aditya,Yomoda, Yuya,Kawagishi, Hirokazu,Terada, Yukimasa,Maoka, Takashi,Etoh, Hideo
, p. 2596 - 2599 (2007)
The reactivity of retinol with peroxynit...
Catalytic activities of tumor-specific human cytochrome P450 CYP2W1 toward endogenous substrates
Zhao, Yan,Wan, Debin,Yang, Jun,Hammock, Bruce D.,De Montellano, Paul R. Ortiz
, p. 771 - 780 (2016)
CYP2W1 is a recently discovered human cy...
Femtosecond transient absorption spectroscopic study of a carbonyl-containing carotenoid analogue, 2-(all- trans -retinylidene)-indan-1,3- dione
Kusumoto, Toshiyuki,Kosumi, Daisuke,Uragami, Chiasa,Frank, Harry A.,Birge, Robert R.,Cogdell, Richard J.,Hashimoto, Hideki
, p. 2110 - 2119 (2011)
The photophysical properties of a carbon...
-
Wald et al.
, p. 438,446 (1955)
-
Sensitized photoisomerization of all-trans- and 11-cis-retinal
Jensen,Wilbrandt,Bensasson
, p. 7877 - 7888 (1989)
The photoisomerization of all-trans-reti...
Retinal-based polyene fluorescent probe for selectively detection of Cu2+ in physiological saline and serum
Li, Yang,Lan, Haichuang,Yan, Xia,Shi, Xiaotao,Liu, Xiao,Xiao, Shuzhang
, (2020)
Retinal is a flexible natural chromophor...
A synthesis method of 9-cis Beta-carotene
-
Page/Page column 0029; 0094-0097, (2021/05/18)
The present invention relates to a metho...
Synthesis of C11-to-C14 methyl-shifted all-: Trans -retinal analogues and their activities on human aldo-keto reductases
Alvarez, Rosana,Barracco, Vito,De Lera, Angel R.,Domínguez, Marta,Farrés, Jaume,Jiménez, Rafael,López, Susana,Parés, Xavier,Pequerul, Raquel,Rivas, Aurea
, p. 4788 - 4801 (2020/07/13)
Human aldo-keto reductases (AKRs) are en...
Mimicking light-sensing chromophore in visual pigments and determination isomerization site
Li, Yang,Lan, Haichuang,Yan, Xia,Shi, Xiaotao,Liu, Xiao,Xiao, Shuzhang
, (2020/01/02)
Three retinal derivatives are designed a...
116-31-4 Process route
-
- 7235-40-7
beta-carotene

-
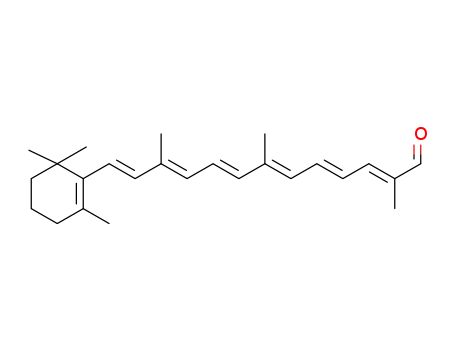
- 1638-05-7
12'-apo-β-caroten-12'-al

-
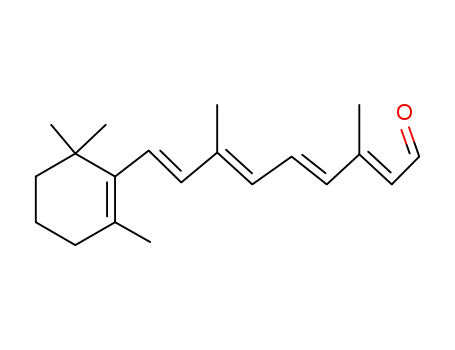
- 116-31-4
all-trans-Retinal

-

- 640-49-3
4,9,13-trimethyl-15-(2,6,6-trimethylcyclohex-1-enyl)pentadeca-2,4,6,8,10,12,14-heptaenal
| Conditions | Yield |
|---|---|
|
With tert.-butylhydroperoxide; Ru[5,15-bis(tolyl)-10,20-bis(6-O-β-cyclodextryl)porphyrin]; In hexane; chloroform; water; for 24h;
|
-
![(S)-2-[(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(E)-ylideneamino]-4-methylsulfanyl-butyric acid](/upload/2024/7/68d16f39-f340-43ce-857c-30e160f38bf5.png)
-
(S)-2-[(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(E)-ylideneamino]-4-methylsulfanyl-butyric acid

-
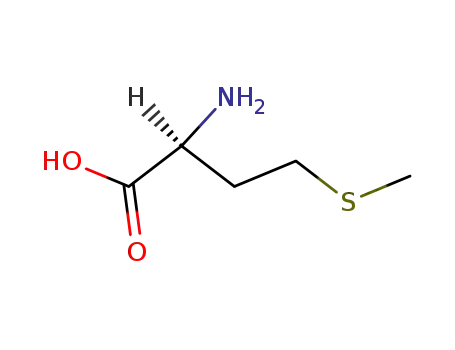
- 63-68-3,26062-47-5,58576-49-1
L-methionine

-

- 116-31-4
all-trans-Retinal
| Conditions | Yield |
|---|---|
|
With phosphate buffer; Rate constant; also hydrolysis in physiological solution and aq. EtOH;
|
116-31-4 Upstream products
-
107-86-8
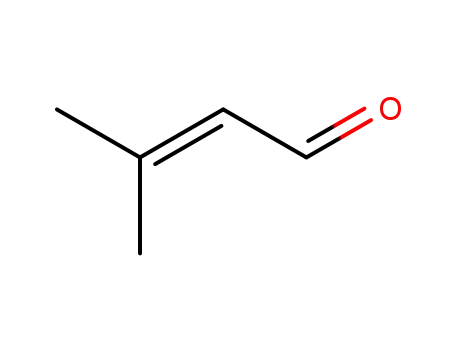
3,3-dimethyl acrylaldehyde
-
3917-41-7
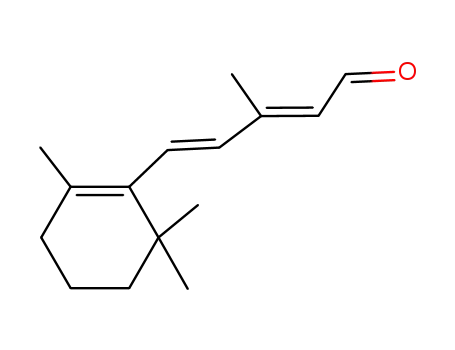
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal
-
7235-40-7

beta-carotene
-
68-26-8
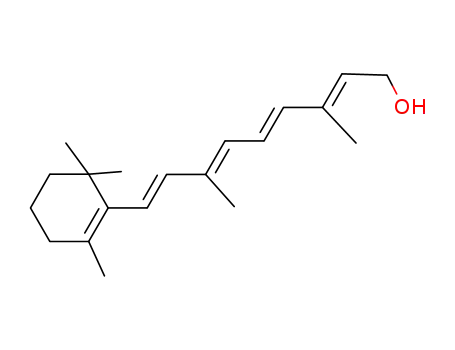
RETINOL
116-31-4 Downstream products
-
121974-71-8
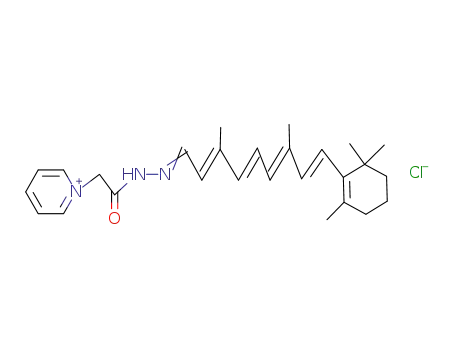
GRP-retinal
-
34218-73-0
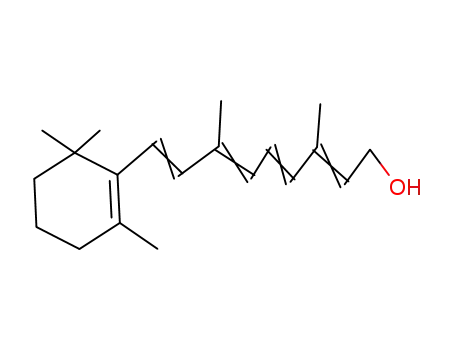
3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nontetraen-1-ol
-
564-87-4
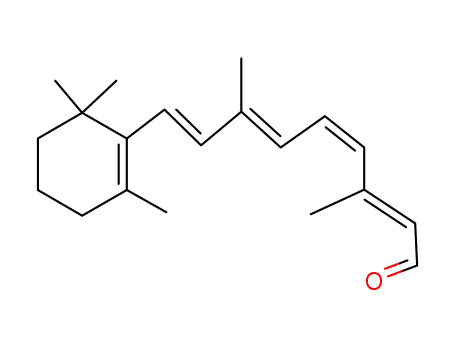
(11Z)-retinal
-
61630-48-6

all-trans-retinal-acetylhydrazone
Relevant Products
-
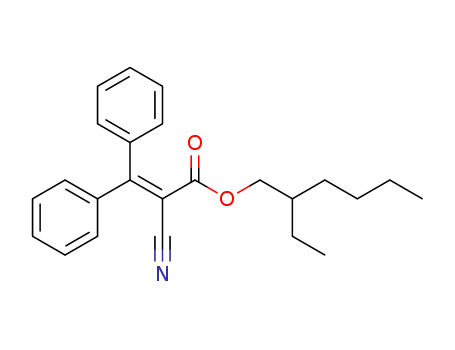
0ctocrylene
CAS:6197-30-4
-
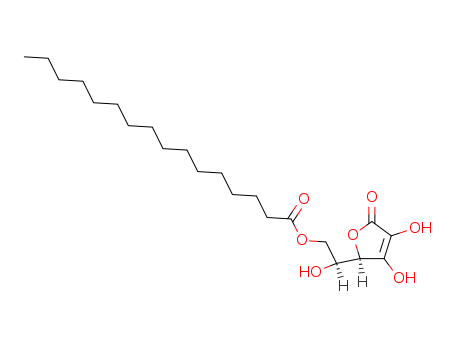
Ascorbyl Palmitate
CAS:137-66-6
-
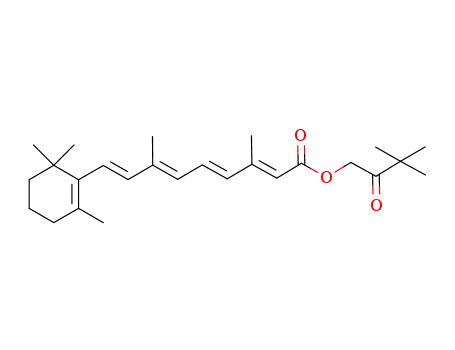
Hydroxypinacolone Retinoate
CAS:893412-73-2