25086-89-9
- Product Name:VP/VA Copolymer
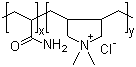
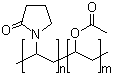
- Molecular Formula:(C6H9NO)n.(C4H6O2)m
- Purity:99%
- Molecular Weight:197.23100
Product Details
Appearance:white powder
High-end VP/VA Copolymer 25086-89-9 top sale around the world
- Molecular Formula:(C6H9NO)n.(C4H6O2)m
- Molecular Weight:197.23100
- Appearance/Colour:white powder
- Vapor Pressure:0.132mmHg at 25°C
- Refractive Index:1.4300 to 1.4380
- Boiling Point:217.6oC at 760 mmHg
- Flash Point:72 °F
- PSA:46.61000
- Density:1.27 g/mL at 25 °C(lit.)
- LogP:1.38320
Poly(1-vinylpyrrolidone-co-vinyl acetate)(Cas 25086-89-9) Usage
|
Production Methods |
Copovidone is manufactured by free-radical polymerization of vinylpyrrolidone and vinyl acetate in a ratio of 6 : 4. The synthesis is conducted in an organic solvent owing to the insolubility of vinyl acetate in water. |
|
Pharmaceutical Applications |
Copovidone is used as a tablet binder, a film-former, and as part of the matrix material used in controlled-release formulations. In tableting, copovidone can be used as a binder for direct compression and as a binder in wet granulation. Copovidone is often added to coating solutions as a film-forming agent. It provides good adhesion, elasticity, and hardness, and can be used as a moisture barrier. |
|
Safety Profile |
Moderately toxic by ingestion.Combustible, especially in powdered form. Incompatiblewith strong oxidising agents, strong reducing agents. Whenheated to decomposition it emits toxic vapors of NOx. |
|
Safety |
Copovidone is used widely in pharmaceutical formulations and is generally regarded as nontoxic. However, it is moderately toxic by ingestion, producing gastric disturbances. It has no irritating or sensitizing effects on the skin. A study was conducted to look at the carcinogenicity and chronic toxicity of copovidone (Kollidon VA 64) in Wistar rats and Beagle dogs. The results of these studies demonstrated the absence of any significant toxicological findings of high dietary levels of copodivone in rats and dogs, resulting in noobserved- adverse-effect levels of 2800 mg/kg body-weight/day in rats and 2500 mg/kg body-weight/day in dogs, the highest doses tested. LD50 (rat, oral): >0.63 g/kg |
|
storage |
Copovidone is stable and should be stored in a well-closed container in a cool, dry place. |
|
Incompatibilities |
Copovidone is compatible with most organic and inorganic pharmaceutical ingredients. When exposed to high water levels, copovidone may form molecular adducts with some materials; see Crospovidone and Povidone. |
|
Regulatory Status |
Copovidone is included in the FDA Inactive Ingredients Database (oral tablets, oral film-coated tablets, sustained action). |
|
General Description |
~1:2.4 mole ratio of VP:VA |
InChI:InChI=1/C6H9NO.C4H6O2/c1-2-7-5-3-4-6(7)8;1-3-6-4(2)5/h2H,1,3-5H2;3H,1H2,2H3
Relevant Products
-
Polyquaternium-7
CAS:26590-05-6
-
Polyvinylpyrrolidone
CAS:9003-39-8
-
Ethyl Ascorbic Acid
CAS:86404-04-8